依折麦布辛伐他汀片对兔腹主动脉粥样硬化斑块逆转作用的实验研究
于熙滢,周大亮,郭 颖,吕 建,魏 林,曹海利
依折麦布辛伐他汀片对兔腹主动脉粥样硬化斑块逆转作用的实验研究
于熙滢1,周大亮1,郭 颖1,吕 建1,魏 林1,曹海利2*
(1哈尔滨市第一医院心内一科,哈尔滨 150001;2哈尔滨医科大学附属第二医院介入科,哈尔滨 150086)
观察腹主动脉粥样斑块内炎性巨噬细胞和平滑肌细胞的表达情况,以探索依折麦布联合他汀类药物在逆转动脉斑块中的作用及机制。选取24只健康雄性新西兰大耳白兔,随机分为对照组(=8)和高胆固醇血症组(=16)。对照组给予普通饲料,喂养12周。高胆固醇血症组喂饲致动脉粥样硬化饲料(由普通颗粒饲料+15g/L胆固醇+100g/L猪油+150g/L蛋黄粉组成)2周后行腹主动脉内膜球囊拉伤术,术后再随机分为模型亚组和依折麦布辛伐他汀(ES)亚组(给予5/10mg/(kg·d)每组8只,两亚组均继续喂饲致动脉粥样硬化饲料10周。喂养第12周时活杀动物,取腹主动脉进行石蜡切片。检测不同时间点脂质和脂蛋白,应用光学显微镜观察动脉粥样硬化进程,采用免疫组化方法分析巨噬细胞和平滑肌细胞在斑块处的表达。ES亚组的血清总胆固醇(TC)、甘油三酯(TG)、低密度脂蛋白胆固醇(LDL-C)浓度明显低于模型亚组(<0.01)。病理检测显示两亚组及ES亚组斑块直径、斑块厚度和动脉内/中膜厚度经单因素方差分析,差异有统计学意义(<0.05)。免疫组化检测结果示ES亚组血管壁中巨噬细胞的表达量较模型亚组显著减少(<0.05),而平滑肌细胞的表达量较模型亚组显著增多(<0.01)。ES可能通过减少细胞外脂质的沉积,减少内膜和中膜巨噬细胞的数量和胆固醇的含量,增加胶原和平滑肌细胞面积,从而起到逆转斑块的作用。
兔;主动脉,腹;巨噬细胞;肌细胞,平滑肌;依折麦布辛伐他汀片
在动脉粥样硬化的发生发展过程中,胆固醇逆向转运的紊乱,血管壁巨噬细胞黏附,平滑肌细胞移行增殖并蓄积脂质形成泡沫细胞是一个重要的环节[1−5]。能有效抑制这一环节并促进细胞内胆固醇逆向转运的药物研发一直是动脉粥样硬化研究关注的热点。依折麦布辛伐他汀(ES)片作为一种新型选择性胆固醇吸收抑制剂与他汀类药物的合剂,在临床应用中因其可有效弥补单独应用他汀类药物的不足而倍受青睐[3,6−9]。但ES片明显降低低密度脂蛋白胆固醇(low density lipoprotein cholesterol,LDL-C)水平能否逆转动脉粥样硬化的进程仍未有定论。本研究拟通过建立家兔动脉粥样硬化模型,观察腹主动脉粥样斑块内炎性巨噬细胞和平滑肌细胞的表达情况,从而探索依折麦布联合他汀类药物在逆转动脉斑块中的作用及机制。
1 材料与方法
1.1 动物模型的建立及分组
雄性新西兰大白兔24只,体质量2.0~2.5kg。将兔单笼喂养普通饲料1周。24只兔随机分为对照组(8只)和高胆固醇血症组(16只)。对照组给予普通饲料。高胆固醇血症组供给致动脉粥样硬化饲料(由普通颗粒饲料+15g/L胆固醇+100g/L猪油+150g/L蛋黄粉组成)。喂养2周后,参照文献[10]的方法进行腹主动脉内膜球囊损伤术。术后再随机分为模型亚组(=8)和ES亚组(=8)。模型亚组继续喂饲致动脉粥样硬化饲料10周。ES亚组除喂饲致动脉粥样硬化饲料加用ES片5/10mg/(kg·d)。喂养第12周时进行腹主动脉造影。并留取腹主动脉左肾动脉水平以下5cm的血管作为标本。所有动物均于实验开始和结束前空腹取每只兔耳缘静脉血测定血清总胆固醇(total cholesterol,TC)、甘油三酯(triglycerides,TG)和高密度脂蛋白胆固醇、LDL-C,进行血液学检查。
1.2 实验材料及试剂
依折麦布辛伐他汀片(固定剂量复方制剂,商品名:葆至能,规格:依折麦布10mg/辛伐他汀20mg,由默沙东−先灵葆雅公司生产,注册证号H20090344)。鼠抗兔巨噬细胞单克隆抗体(RAM11,丹麦DAKO公司),SP免疫组化染色试剂盒(北京中杉金桥生物技术有限公司),胆固醇(武汉亚法生物技术拓展公司进口分装),TC、TG试剂盒(英国Randow公司),LDL-C生化试剂盒(日本第一化学公司)。
1.3 给药剂量、途径及时间
由于辛伐他汀在水中溶解度较差但易溶于乙醇。取适量药品溶解于乙醇,一边添加一边搅拌,并在涡旋仪上震荡2min。并在给药时注意充分使药物混悬液分布均匀。本实验根据相关参考文献[11]以及人与各种动物的剂量折算系数法,并在安全许可剂量范围内我们设计应用ES片5/10mg/(kg·d)剂量。兔经口灌胃,每天傍晚(17∶00~18∶00)给药1次,每次给药体积控制在5~7ml。
1.4 实验方法
1.4.1 药物触发 3组白兔均给予中国斑点蝰蛇毒0.15mg/kg腹膜下注射,30min后,经耳缘静脉注射组胺0.02mg/kg,于活杀动物前24和48h给予两次药物触发斑块破裂。
1.4.2 腹主动脉造影检查 对照组、模型亚组、ES亚组3组动物分别于12周行腹主动脉造影检查,手术切开暴露右股动脉,穿刺针直视下穿刺,留置管鞘,行腹主动脉造影。以Image-Pro Plus6.0软件精确测量每根腹主动脉狭窄区的最小残腔直径(diameter of minimum cavity,DMC)和狭窄远端腹主动脉直径(diameter of distal abdominal,DDA)。狭窄率=(DDA-DMC)/DDA×100%。狭窄程度的判定标准:狭窄率<30%为轻度狭窄,30%~69%为中度狭窄,70%~99%为重度狭窄,100%为完全闭塞。
1.5 病理标本的留取
1.5.1 标本大体形态观察 动物活杀后取出腹主动脉全长,纵行切开,生理盐水冲洗干净后,观察斑块形成情况。然后以4%甲醛固定,同时用HE染色整条主动脉,进行病理切片后经Image-Pro Plus6.0分析系统处理,计算斑块直径、斑块厚度、内/中膜厚度。
1.5.2 免疫组织化学检查 选取邻近主动脉弓部的降主动脉,置于4%甲醛固定48h,常规石蜡包埋,血管横断面作4μm连续切片,分别做HE染色及巨噬细胞和平滑肌肌动蛋白的免疫化学染色。应用RAM11抗体和α-肌动蛋白(actin)抗体分别测定斑块破裂与未破裂部位的巨噬细胞和平滑肌肌动蛋白的局部表达。
1.6 统计学处理
2 结 果
2.1 各组兔血脂变化情况
在喂养过程中,模型亚组死亡2只,ES亚组死亡1只。实验前各组兔血清TC、TG、LDL-C比较,差异无统计学意义(>0.05)。与实验前比较,对照组12周后TC、TG、LDL-C无明显变化(>0.05),而两亚组12周后血清TC、TG、LDL-C较实验前明显升高(<0.01),并且高于对照组(<0.01),ES亚组血清TC、TG、LDL-C较模型亚组明显下降(<0.01;表1)。
2.2 腹主动脉狭窄程度
对照组无狭窄。两亚组均有不同程度的狭窄,模型亚组多为中重度狭窄,无完全闭塞者。ES亚组多为轻度狭窄。
2.3 病理形态学观察
对照组兔的动脉内皮细胞完整,无明显脂质沉积(图1A)。模型亚组部分斑块中有新生血管,并有大量的炎症细胞浸润,多数血管可见粥样斑块或纤维斑块,内膜下大量胞浆淡染和充满脂质空泡的泡沫细胞,弹力纤维崩解、断裂、溶解,斑块纤维帽薄,胶原少,呈典型的易损斑块(图1B)。ES亚组动脉壁内膜厚度明显小于模型亚组,内膜完整,泡沫细胞减少,脂质沉积少(图1C)。
2.4 斑块直径、厚度及内/中膜厚度
病理检测显示两亚组12周时斑块直径、斑块厚度、内/中膜厚度,经单因素方差分析表明,差异有统计学意义(<0.01;表2)。
2.5 免疫组织化学分析
经Image-Pro Plus6.0分析系统处理,对照组缺乏巨噬细胞表达,可见中层平滑肌细胞着色,无内膜增生(图2A,3A)。以斑块纤维帽500μm×100μm的面积区域计数细胞数。结果显示与ES亚组相比,模型亚组巨噬细胞的数量较之明显增多(46.4±5.638.6±6.4),差异具有统计学意义(<0.05;图2B,2C);模型亚组平滑肌细胞较ES亚组明显减少(57.6±6.371.2±4.9),差异有统计学意义(<0.01;图3B,3C)。
3 讨 论
动脉粥样硬化的典型病理学特征是动脉壁内皮下间隙脂质沉积、内膜增厚乃至最终出现血管腔的狭窄。许多证据表明,单核巨噬细胞的黏附和平滑肌细胞的内膜迁移在动脉粥样硬化的发生发展中具有重要作用[12−15]。如何有效地抑制甚或逆转泡沫细胞(巨噬细胞、平滑肌细胞吞噬脂质成为泡沫细胞)的形成成为防治动脉粥样硬化的关键。实验研究已证实,高脂血症可引起单核巨噬细胞发生功能改变增进黏附进入动脉内膜的能力,同时又能促进平滑肌细胞向内膜移动。本研究显示,动脉粥样斑块中巨噬细胞明显增多,模型亚组巨噬细胞表达较ES亚组显著增多,说明炎症反应促成了腹主动脉粥样硬化的形成,并可能进一步导致斑块的不稳定。

表1 兔实验前后血脂水平
TC: total cholesterol; TG: triglycerides; LDL-C: low density lipoprotein cholesterol; ES: ezetimibe/simvastatin. Compared with control group,**<0.01; compared with model subgroup,##<0.01; compared with the beginning of the experiment in the same group,△△<0.01

图1 兔腹主动脉HE染色结果
Figure 1 Hematoxylin-eosin staining results of rabbits abdominal aorta (×40) A: control group; B: model subgroup; C: ES subgroup

图2 巨噬细胞免疫组化染色结果
Figure 2 Results of immunohistochemical staining in macrophages(×400) A: control group; B: model subgroup; C: ES subgroup
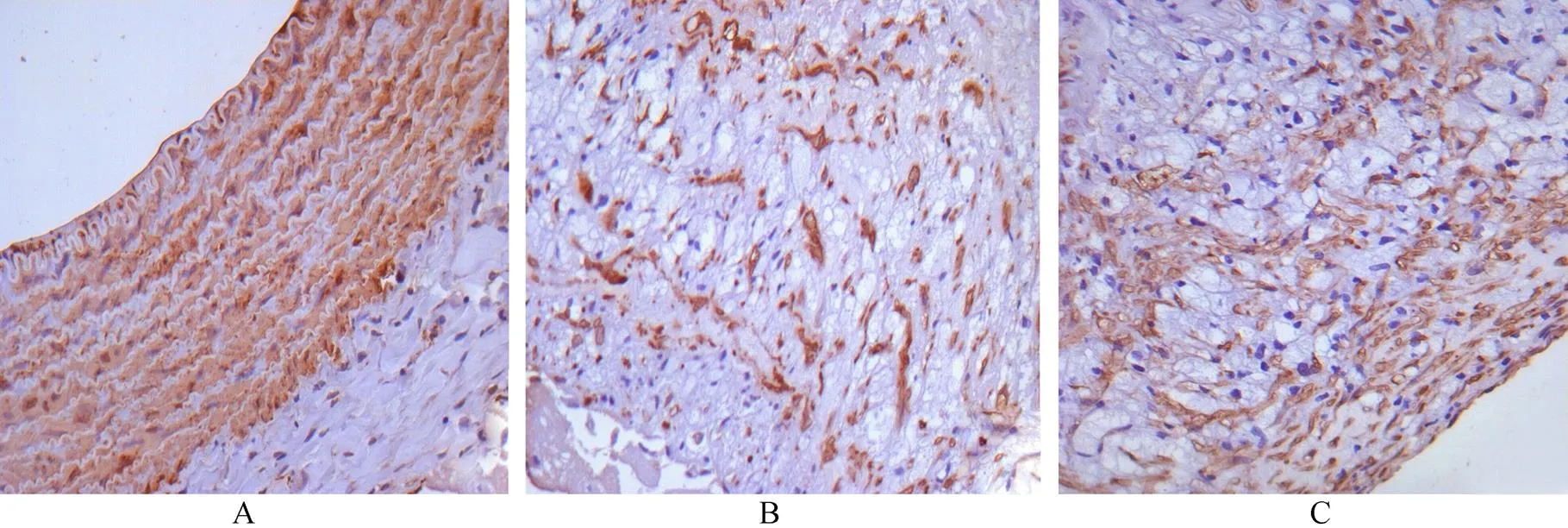
图3 平滑肌肌动蛋白免疫组化染色结果
Figure 3 Results of immunohistochemical staining in smooth muscle actin (×400) A: control group; B: model subgroup; C: ES subgroup
表2 两亚组斑块直径、厚度、内-中膜厚度比较
Compared with model subgroup,**<0.01
依折麦布是一种选择性胆固醇吸收抑制剂,其作用机制主要是阻断胆固醇的外源性吸收途径。其口服后被迅速吸收且广泛结合成依折麦布-葡萄糖苷酸,作用于小肠细胞的刷状缘,通过抑制C型1类尼曼匹克−C1样蛋白l(Niemann-Pick C1 like 1,NPC1L1),选择性地抑制膳食和胆汁中的胆固醇跨小肠壁转运,有效地阻止胆固醇和植物固醇的吸收。一项单药治疗的研究[16]显示,10mg依折麦布单药治疗2周后,LDL-C较基线水平降低了20.4%,安慰剂组则升高了1.9%;组间相比,LDL-C降幅相差22.3%。辛伐他汀作为一种羟甲基戊二酰辅酶A还原酶抑制剂,能特异性地降低肝细胞中胆固醇的合成,进而发挥降低血脂的作用。两者联合应用可分别从胆固醇的内、外源性途径对血脂水平进行调节以达到最佳调脂效果[17]。本研究动物实验显示,连续12周5/10mg/(kg·d)的ES片可有效减轻新西兰兔主动脉粥样硬化的病变程度,内/中膜变薄,内皮下间隙脂质以及坏死物质减少,血管壁结构得到了有效的改善。本研究中ES亚组显著降低高胆固醇血症家兔血清TC、TG和LDL-C浓度,同时,还显著抑制了腹主动脉粥样斑块的形成,该亚组斑块近心端巨噬细胞和表达的数目较模型组明显减少,减轻了斑块近心端细胞外基质的降解,使斑块趋向稳定。
本研究的局限性在于样本量较小,且观察时间较短。只设有ES片两者组成的固定复方制剂ES组,未设有与依折麦布单药及辛伐他汀单药组的疗效对照研究。在后续研究中,将扩大样本量,延长观察时间,通过与单药治疗对比以观察ES片对远期心脏事件的影响。总之,ES片的治疗可稳定动脉粥样硬化斑块,包括可减少细胞外脂质的沉积,减少内膜和中膜巨噬细胞的数量和胆固醇的含量,增加胶原和平滑肌细胞面积,减少内膜的钙化和新生血管,通过减少血中LDL-C水平,缩小斑块内脂核,减少斑块表面张力,加固斑块纤维帽,最大限度地稳定斑块并使其退缩,具有较强的抗增殖作用和抗迁移作用。
[1] Stein EA, Vidt DG, Shepherd J,. Renal safety of intensive cholesterol-lowering treatment with rosuvastatin: a retrospective analysis of renal adverse events among 40 600 participants in the rosuvastatin clinical development program[J]. Atherosclerosis, 2012, 221(2): 471−477.
[2] Lee CW, Kang SJ, Ahn JM,. Comparison of effects of atorvastatin (20mg)rosuvastatin (10mg) therapy on mild coronary atherosclerotic plaques (from the ARTMAP trial)[J]. Am J Cardiol, 2012, 109(12): 1700−1704.
[3] Inazawa T, Sakamoto K, Kohro T,. RESEARCH (Recognized Effect of Statin and Ezetimibe Therapy for achieving LDL-C Goal), a randomized, doctor-oriented, multicenter trial to compare the effects of higher-dose statinezetimibe-plus-statin on the serum LDL-C concentration of Japanese type-2 diabetes patients design and rationale[J]. Lipids Health Dis, 2013, 12:142.
[4] Yan YL, Qiu B, Hu LJ,. Efficacy and safety evaluation of intensive statin therapy in older patients with coronary heart disease: a systematic review and meta-analysis[J]. Eur J Clin Pharmacol, 2013, 69(12): 2001−2009.
[5] Zheng JS, Yang J, Huang T,. Effects of Chinese liquors on cardiovascular disease risk factors in healthy young humans[J]. Sci World J, 2012, 2012: 372143.
[6] Tani S, Nagao K, Hirayama A. HMG-CoA reductase inhibitor (statin) therapy and coronary atherosclerosis in Japanese subjects: role of high-density lipoprotein cholesterol[J]. Am J Cardiovasc Drugs, 2011, 11(6): 411−417.
[7] Anogeianaki A, Angelucci D, Cianchetti E,. Atherosclerosis: a classic inflammatory disease[J]. Int J Immunopathol Pharmacol, 2011, 24(4): 817−825.
[8] Kashiwagi M, Kitabata H, Ozaki Y,. Fatty streak assessed by optical coherence tomography: early atherosclerosis detection[J]. Eur Heart J Cardiovasc Imaging, 2013, 14(2): 109.
[9] Yunoki K, Nakamura K, Miyoshi T,. Impact of hypertriglyceridemia on endothelial dysfunction during statin ezetimibe therapy in patients with coronary heart disease[J]. Am J Cardiol, 2011, 108(3): 333−339.
[10] Constantinides P, Chakravarti RN. Rabbit arterial thrombosis production by systemic procedures[J]. Arch Pathol, 1961, 72: 197−208.
[11] Igel M, Arnold KA, Niemi M,. Impact of the SLCO1B1 polymorphism on the pharmacokinetics and lipid-lowering efficacy of multiple-dose pravastatin[J]. Clin Pharmacol Ther, 2006, 79(5): 419−426.
[12] Hyafil F1, Cornily JC, Rudd JH,. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with18F-FDG PET/CT and histology[J]. J Nucl Med, 2009, 50(6): 959−965.
[13] D’Ascenzo F, Agostoni P, Abbate A,. Atherosclerotic coronary plaque regression and the risk of adverse cardiovascular events: a meta-regression of randomized clinical trials[J]. Atherosclerosis, 2013, 226(1): 178−185.
[14] Shanmugam N, Román-Rego A, Ong P,. Atherosclerotic plaque regression: factor fiction[J]? Cardiovasc Drugs Ther, 2010, 24(4): 311−317.
[15] Macías Saint-Gerons D, Dela Fuente Honrubia C, Montero Corominas D,. Standard and intensive lipid-lowering therapy with statins for the primary prevention of vascular diseases: a population-based study[J]. Eur J Clin Pharmacol, 2014, 70(1): 99−108.
[16] Sudhop T, Lutjohann D, Kodal A,. Inhibition of intestinal cholesterol absorption by ezetimibe in humans[J]. Circulation, 2002, 106(15): 1943
[17] Mikhailidis DP, Lawson RW, McCormick AL,. Comparative efficacy of the addition of ezetimibe to statinstatin titration in patients with hypercholesterolaemia: systematic review and meta-analysis[J]. Curr Med Res Opin, 2011, 27(6): 1191−1210.
(编辑: 周宇红)
Effects of ezetimibe/simvastatin tablets on atherosclerotic plaque regression of abdominal arteries in rabbits
YU Xi-Ying1, ZHOU Da-Liang1, GUO-Ying1, LYU- Jian1, WEI-Lin1, CAO Hai-Li2*
(1Department No.1 of Cardiology, Harbin First Hospital, Harbin 150010, China;2Department of Interventional Radiology, the Second Affiliated Hospital, Harbin Medical University, Harbin 150086, China)
To observe the efficiency of ezetimibe/simvastatin (ES) tablets on the regression of atherosclerotic plaque of abdominal arteries in rabbits.Twenty-four healthy male New Zealand rabbits were randomly divided into 2 groups: control group (=8) and hypercholesterolemia group (=16). Control group was fed with normal diet for 12 weeks. The other group animals were given a cholesterol-supplemented diet (normal diet+15g/Lcholesterol+100g/Llard+150g/Legg yolk powder) for 2 weeks, and underwent catheter-induced arterial wall injury. These rabbits were then randomized to model subgroup (=8, for another 10 weeks of hypercholesterol diet) and ES treatment subgroup [=8, 5/10mg/(kg·d) for another 10 weeks]. Chinese russell’s viper venom was intra-peritoneally injected to trigger plaque rupture. Abdominal aortagraphy was carried out to measure the aorta stenosis. After 12 weeks’ feeding, all rabbits were sacrificed, and their abdominal arteries were isolated, paraffin-embedded and then sectioned. Blood lipid and lipoproteins were detected. The development of the atherosclerotic plaques was evaluated through the light microscopy. Finally, the expression of macrophage and smooth muscle actin in the abdominal arteries was measured by immunohistochemical analysis.The serum levels of total cholesterol (TC), triglycerides (TG) and low density lipoprotein-cholesterol (LDL-C) were significantly lower in the ES treatment subgroup than in the hypercholesterolemia model group (<0.01). One-way analysis of variance indicated that significant differences were found in the plaque diameter, plaque thickness and the intimal-medial thickness between the ES subgroup and hypercholesterolemia model subgroup by morphological observation (<0.05). Immunohistochemical analysis showed that lesser macrophages (<0.05) but more smooth muscle cells (<0.01) were found in the ES treatment subgroup than in the model group.It may be through reducing the deposition of extracellular lipids that ES treatment decreases macrophage number and cholesterol level, increases the collagen and smooth muscle cells in the arterial intima and media, and thus exerts effect on plaque reversing.
rabbits; aorta, abdominal; macrophage; myocyte, smooth muscle; ezetimibe/simvastatin tablets
R543.1+2
A
10.3724/SP.J.1264.2014.000214
2014−08−24;
2014−10−21
曹海利, E-mail: 18746013637@163.com

