Oxidative phosphorylated neuro fi lament protein M protects spinal cord against ischemia/reperfusion injury
Haitao Wang, Su Pan, Xiaoyu Yang, Benqing Zhu, Dalin Wang
1 Department of Orthopedic Surgery, Af fi liated Hospital of Beihua University, Jilin, Jilin Province, China
2 Department of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics, People’s Hospital of Tianjin, Tianjin, China
Oxidative phosphorylated neuro fi lament protein M protects spinal cord against ischemia/reperfusion injury
Haitao Wang1, Su Pan2, Xiaoyu Yang2, Benqing Zhu3, Dalin Wang1
1 Department of Orthopedic Surgery, Af fi liated Hospital of Beihua University, Jilin, Jilin Province, China
2 Department of Orthopedics, the Second Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Orthopedics, People’s Hospital of Tianjin, Tianjin, China
Previous studies have shown that neuro fi lament protein M expression is upregulated in the early stage of spinal cord ischemia/reperfusion injury, indicating that this protein may play a role in the injury process. In the present study, we compared protein expression in spinal cord tissue of rabbits after 25 minutes of ischemia followed by 0, 12, 24, or 48 hours of reperfusion with that of sham operated rabbits, using proteomic two-dimensional gel electrophoresis and mass spectrometry. In addition, the nerve repair-related neurofilament protein M with the unregulated expression was detected with immunohistochemistry and western blot analysis. Two-dimensional gel electrophoresis and mass spectrometry showed that, compared with the sham group, upregulation of protein expression was most signi fi cant in the spinal cords of rabbits that had undergone ischemia and 24 hours of reperfusion. Immunohistochemical analysis revealed that neuro fi lament protein M was located in the membrane and cytoplasm of neuronal soma and axons at each time point after injury. Western blot analysis showed that neuro fi lament protein M expression increased with reperfusion time until it peaked at 24 hours and returned to baseline level after 48 hours. Furthermore, neuro fi lament protein M is phosphorylated under oxidative stress, and expression changes were parallel for the phosphorylated and non-phosphorylated forms. Neurofilament protein M plays an important role in spinal cord ischemia/reperfusion injury, and its functions are achieved through oxidative phosphorylation.
nerve regeneration; neurofilament protein M; spinal cord injury; ischemia/reperfusion; proteomics; phosphorylation; neuroprotection; NSFC grant; neural regeneration
Funding:This study was financially supported by the National Natural Science Foundation of China, No. 81350013, 30872609.
Wang HT, Pan S, Yang XY, Zhu BQ, Wang DL. Oxidative phosphorylated neurofilament protein M protects spinal cord against ischemia/reperfusion injury. Neural Regen Res. 2014;9(18):1672-1677.
Introduction
Many proteins are involved in spinal cord ischemia/reperfusion (I/R) injury, affecting neuronal survival, axonal regeneration and reconstruction of functional synaptic connections in the injured spinal cord. In preliminary studies, we successfully identi fi ed differentially expressed proteins in spinal cord tissue after I/R injury and found that the expression of neuro fi lament protein M (NF-M) was upregulated, indicating that NF-M is involved in the spinal cord I/R injury process. Neuro fi lament expression is closely related to axonal outgrowth and maintenance of neuronal homeostasis (Wang et al., 2012). In the brain, neuro fi lament expression maintains axonal growth under normal conditions as well as in injured nerve tissue (Kuhle et al., 2014). In addition, phosphorylated NF is responsible for regulating a variety of biological functions such as axonal transport in neurons (Lee et al., 2011). NF phosphorylation contributes signi fi cantly to microtubule stabilization in axons, promoting axonal regeneration (Lobsiger et al., 2005). The aim of the present study was to identify protein expression changes in rabbits with spinal cord I/R injury and explore the correlation between differentially expressed proteins and I/R injury, using immunohistochemistry and western blot analysis.
Materials and Methods
Experimental animals
Thirty male and female clean grade New Zealand white rabbits, 7 months old and weighing 2.30 ± 0.40 kg, were provided by the Laboratory Animal Centre of Jilin University, China (license No. SCXK (Ji) 2011-0006). All rabbits were allowed free access to water and food, and were housed in individual cages at 20°C. The investigations conformed tothe Guide for the Care and Use of Laboratory Animalspublished by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee from Jilin University in China. Rabbits were randomly divided into the following groups, each containing six rabbits: sham, I25minR0, I25minR12h, I25minR24h and I25minR48h.
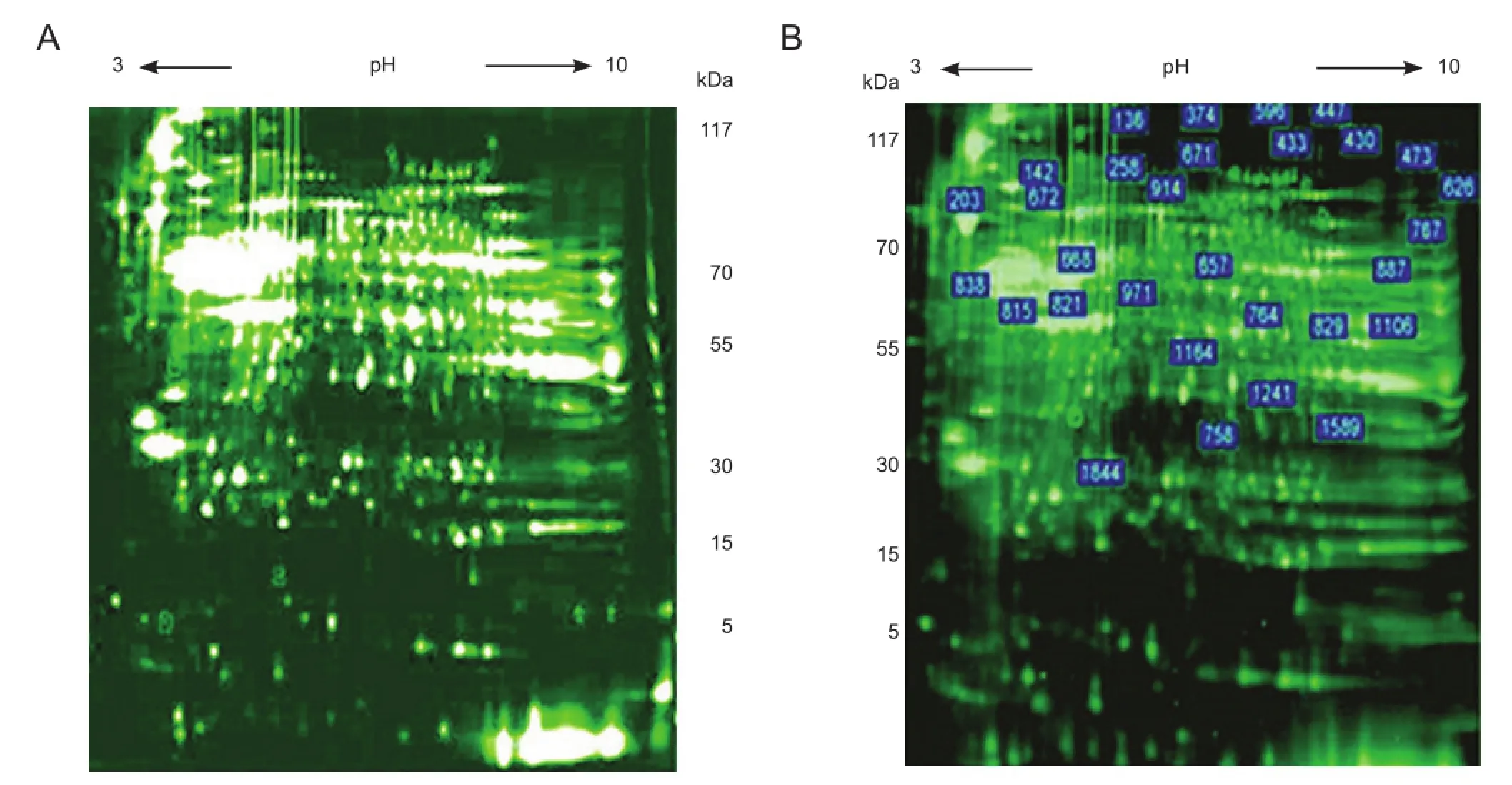
Figure 1 Two-dimensional fl uorescence electrophoresis analysis comparing protein expression in rabbits with and without spinal cord ischemia/reperfusion (I/R) injury.
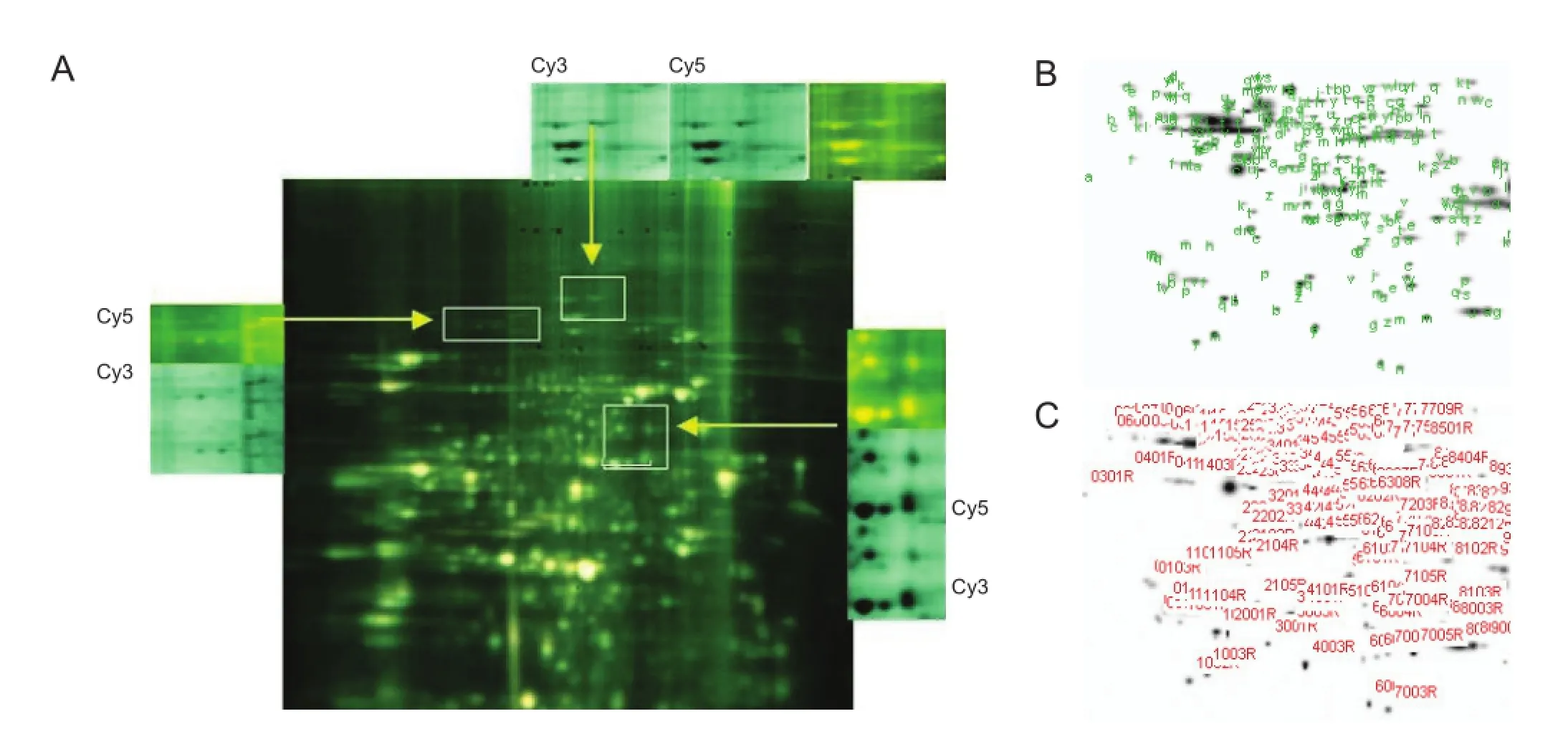
Figure 2 Protein fi ngerprinting.
Establishment of spinal cord I/R injury models
Rabbits were anesthetized with xylazine hydrochloride (0.25 mL/kg; Founder Animal Pharmacy, Changchun, China) injected into the thigh muscle, and the lumbar artery was exposedviathe retroperitoneal approach. Under an operating microscope, the artery at lumbar segments 3-5 (L3-5) was blocked for 25 minutes with a vascular clamp and then released. The artery was inspected to ensure that no damage was found, before being revascularized and the arterial pulse was recovered at the distal end of the lumbar artery. The surgical area was sterilized using gentamicin, the abdominal cavity was closed and the wound was sutured. No deathsoccurred from arterial injury during the surgery. Once the spinal cord I/R injury model was successfully established, animals were housed individually in a well-ventilated room at 20°C. Artificial abdominal compression was performed to assist defecation. One model rabbit died after surgery from an overdose of anesthesia. When the animals were fully awake after anesthesia, bilateral hindlimb motor functions were evaluated and scored by three physicians blind to experimental treatment, according to Tarlov’s method (Peştean et al., 2013). The fi nal score was the mean of three recordings, and the animals that scored 2-3 points were used in this study. A high success rate of spinal cord I/R injury model establishment was achieved with the method used. In the sham group, the abdominal aorta was exposed for 25 minutes with no arterial occlusion and the rabbits were killed for tissue analysis. In the I25minR0 group, the rabbits were killed immediately after the 25 minutes of lumbar artery occlusion, with no reperfusion; in the I25minR12h, I25minR24h and I25minR48h groups, the rabbits were killed after 12, 24, and 48 hours of reperfusion, respectively. L3-5arteries were then harvested, frozen in liquid nitrogen and stored at -80°C until use.
Extraction of soluble proteins from spinal cord
The spinal cord tissue was weighed, and 2-4 mL lysate per gram was added. The proteins were extracted using sonication and the liquid nitrogen freeze-thaw method, then centrifuged at 40,000 r/min for 30 minutes, and stored at -70°C. Protein concentration was determined using the Bradford method (Bradford, 1976). After excess liquid was absorbed using fi lter paper, the specimens were weighed and preserved in liquid nitrogen.
Protein isolation using two-dimensional fl uorescent gel electrophoresis
The proteins were labeled and the fi rst dimension of two-dimensional SDS gel electrophoresis was performed using an IPGphorTMisoelectric focusing system, as described previously (Zhu et al., 2010) and according to the manufacturer’s instructions. Vertical SDS-PAGE electrophoresis was performed using a 17 cm pH3-10 NL IPG strip. The specimens were stained with Coomassie blue R350.
Gel image analysis
Cy3 and Cy5 fl uorescent dye-labeled images were digitized with a transmission scanner (Typhoon 9410; GE Amersham Biosciences, Santa Clara, CA, USA). The resulting spectrum was analyzed using DeCyder v.5.02 software (GE Amersham Biosciences) and the protein spots were compared after image background subtraction, point testing and matching. Proteins with more than twofold difference in expression level were analyzed with mass spectrometry.
Identi fi cation of proteins using peptide mass fi ngerprinting
The differentially expressed proteins were cut from the gel, the gel pieces were destained, and the matrix was dissolved by adding 100 μL acetonitrile. The samples were freeze dried at 20°C for 20 minutes, then incubated in 0.01 μg/μL enzyme solution at 37°C overnight. They were lyophilized in 100 μL of 5% trifluoroacetic acid at -20°C, before 0.6 μL of each sample was injected into a TofSpec re fl ection matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF-MS; Thermo Finnigan, San Jose, USA) using NH4-acetic acid buffer and dithiothreitol. Reflection scanning was performed using MALDI-TOF-MS (Merlos Rodrigo et al., 2014) in the 700-4,000 Da range, with the laser energy set at 5,000 V and 5,500 V. The matched proteins were identified in the NCBInr database (prowl.rockefeller. edu/cgi-bin/ProFound).
NF-M immunohistochemistry
Rabbit L3-5spinal cord specimens were stained with hematoxylin-eosin and made into paraffin sections, which were then dewaxed, hydrated, and antigen-retrieved. The sections were then incubated with rat anti-rabbit NF-M monoclonal antibody (1:150; Invitrogen, Carlsbad, CA, USA) at 37°C for 2 hours, and rinsed with PBS three times for 5 minutes each time, before being incubated with horseradish peroxidase-conjugated goat anti-rat IgG (1:50; Abcam, Cambridge, UK) and peroxidase complex at room temperature for 30 minutes. After three PBS washes, the sections were developed using DAB and mounted with neutral gum, and the brown positive-stained particles were counted in six randomly selected high-power fi elds under an optical microscope (Olympus, Nagano, Japan). The percentage of positive cells was calculated and graded according to a 5-level scale:< 10% (-); 10-25% (±); 25-50% (+); 50-75% (++); > 75% (+++).
NF-M western blot analysis
Rabbit L3-5spinal cord tissue was frozen in liquid nitrogen until use. The tissue was homogenized ultrasonically in 400 μL protease inhibitor cocktail (Abcam, Cambridge, UK). The homogenate was centrifuged at 4°C for 5 minutes at 12,000 r/min, and stored at -20°C after the supernatant was discarded. Protein concentration was measured using Coomassie blue staining. A 15% separating gel was prepared and injected into a vertical glass sandwich, and a 5% stacking gel was added on top. The gels were placed in the electrophoresis tank, and samples containing 50 μg proteins or 10 μL pre-staining marker were loaded for electrophoresis. The proteins were transferred to nitrocellulose membranes at 100 V, 250 mA, 4°C for 2.5 hours, and blocked with fetal bovine serum for 1 hour. The samples were then incubated with rat anti-rabbit NF-M monoclonal antibody (1:150; Invitrogen) and rat anti-rabbit GAPDH monoclonal antibody (1:150; EarthOx, San Francisco, CA, USA) at room temperature for 2 hours, and rinsed with Tris buffered saline + Tween 20 twice for 10 minutes each, and with Tris buffered saline for 10 minutes. Finally, the samples were incubated with goat anti-rat IgG (1:3,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for 1 hour. The protein signal was visualized using an ECL kit (Invit-rogen) and Quantity One Software (Bio-Rad, Hercules, CA, USA) was used to quantify the protein band density (optical density) on the X-ray fi lms. The expression level of NF-M was calculated as the optical density ratio of NF-M to GAPDH.
Statistical analysis
Data were expressed as the mean ± SEM and analyzed with one-way analysis of variance using SPSS 13.0 software (SPSS, Chicago, IL, USA).P< 0.05 was considered statistically signi fi cant.
Results
Differentially expressed proteins in rabbits with spinal cord I/R injury detected by two-dimensional gel image analysis
Two-dimensional fluorescent gel electrophoresis showed that, compared with the sham group, the greatest differences in fluorescence occurred in spinal cord tissue from the I25minR24h group, in which 29 proteins were differentially expressed (Figure 1). Expression was lower in 26 protein spots and more than twofold greater in the remaining three proteins. These three proteins were cut from the gels (Figure 2A) and their peptide fragment sequences were analyzed and screened in the NCBI and rabbit protein databases (Figure 2B, C). They were identi fi ed as NF-M, α-tubulin and fascin actin-bundling protein 1 (FASCN1).
NF-M expression in rabbit spinal cord after I/R injury
Immunohistochemical analysis showed that NF-M expression in the spinal cord tissue was twofold greater in the I25minR0h and I25minR48h groups than in the sham group, threefold greater in the I25minR12h group, and fourfold greater in the I25minR24h group (Figure 3A-E), consistent with proteomics results. NF-M expression reached a peak after 24 hours of reperfusion. NF-M was expressed in somal and axonal membranes (Figure 3F), and the percentage of immunopositive cells in the gray matter was significantly higher than that in the white matter, but there was no significant difference between anterior and posterior horn (data not shown).
NF-M phosphorylation in spinal cord tissue of rabbits after I/R injury
Western blot analysis revealed that NF-M protein expression was upregulated in the I/R process, peaking after 24 hours of reperfusion, before decreasing. The phosphorylated NF-M proteins showed similar expression changes (Figure 4), peak expression also occurring at 24 hours of reperfusion (P<0.05). Previous proteomics studies found that upregulated NF-M was shifted transversely, at the same molecular weight level, indicating that the protein was phosphorylated in the spinal cord injury process (de Groot et al., 2006). Our fi ndings suggest that NF-M proteins are neuroprotective in rabbits with spinal cord I/R injury, and that this protective effect is achieved through NF-M phosphorylation.
Discussion
NF-M is a major component in the mature neuronal cytoskeleton (Carter et al., 1998). It also plays an important role in the development and maturation of the nervous system, and is involved in neuronal recovery in neurodegenerative diseases (Blizzard et al., 2013). However, the role of NF-M in spinal cord I/R injury is rarely reported. Compared with the traditional two-dimensional electrophoresis method, two-dimensional fluorescence gel electrophoresis is more reliable in identifying differentially expressed proteins, owing to small sample consumption, good repeatability, high matching rate, sensitive signals and easy image presentation (Tannu and Hemby, 2006). The present study showed that NF-M expression in spinal cord tissue was notably upregulated 24 hours after reperfusion began, indicating that NF-M is involved in the spinal cord I/R injury process, in particular at 24 hours post-reperfusion. In two-dimensional gel electrophoresis, NF-M was located at different positions within the same molecular weight level, suggesting that NF-M was phosphorylated in the spinal cord I/R injury process (Deng et al., 2008). Previous studies demonstrated that phosphorylation of NF proteins was associated with neuronal regulation and played an important role in NF translocation, morphology and function (Minoura et al., 2013; Xiong et al., 2013). Wataya et al. (2002) highlighted the importance of phosphorylated NF-M in protecting the neurites in rat sciatic nerve, and Zhu et al. (2002) showed that NF-M is involved in axonal growth.
Immunohistochemistry is a sensitive method for the localization of target proteins. Using immunohistochemistry, we showed that the percentage of NF-M positive particles was greatest 24 hours after reperfusion began, supporting the results obtained in our proteomics analysis. NF-M positive particles were mainly distributed in the gray matter of the spinal cord, and located in the membrane and cytoplasm of neuronal cell bodies and axons. The distribution of NF-M was not signi fi cantly different between the anterior and posterior horns. Together, this evidence indicates that the upregulation of NF-M expression contributes to the protection and restoration of spinal cord neurons and axons during the reperfusion process after ischemia.
Consistent with our proteomics and immunohistochemistry results, western blot analysis demonstrated that NF-M expression was greatest at 24 hours of reperfusion. Expression of phosphorylated NF-M was the same as that of unmodi fi ed NF-M in the spinal cord I/R injury process. Neurofilament phosphorylation plays an important role in neuro fi lament translocation, morphology and function, and is also involved in the pathogenesis of some neurodegenerative diseases (Holmgren et al., 2012). Tashiro et al. (1994) found that neurofilament phosphorylation stabilized axonal microtubules, and thus promoted axonal regeneration in the rat sciatic nerve. Taking into consideration our present results, we speculate that phosphorylation of NF-M is the mechanism by which this important protein exerts its neuroprotective effects in spinal cord I/R injury.
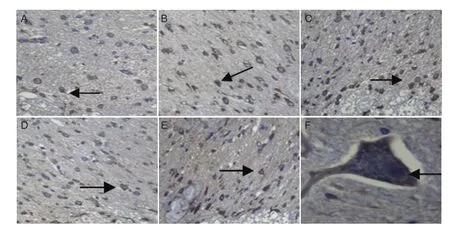
Figure 3 Neuro fi lament protein M expression in rabbit spinal cord after ischemia/reperfusion (I/R) injury (immunohistochemistry).
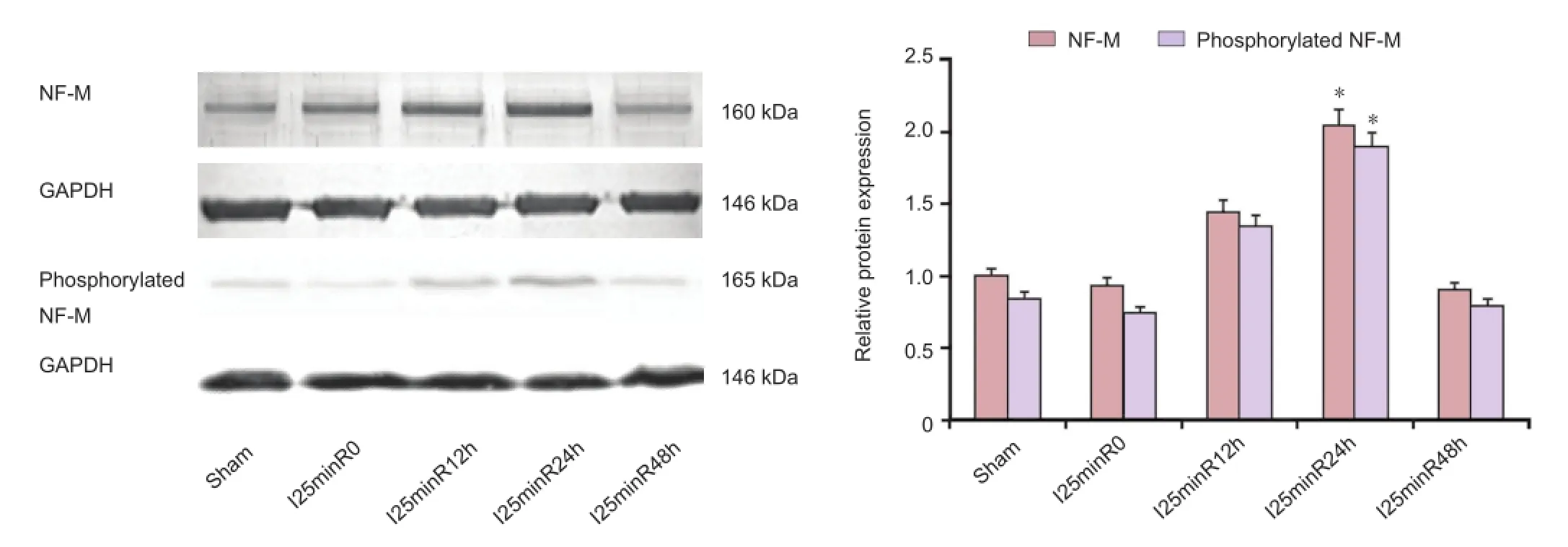
Figure 4 Neuro fi lament protein M (NF-M) phosphorylation in rabbit spinal cord tissue after ischemia/reperfusion (I/R) injury.
In summary, using proteomics, immunohistochemistry and western blot analysis, we provide evidence supporting a newly discovered role of NF-M phosphorylation in spinal cord I/R injury.
Author contributions:Wang HT was responsible for data acquisition, integration and analysis, and writing the article. Pan S performed statistical analysis of experimental data. Yang XY was responsible for the study concept and design, instructed the study and validated the article. Zhu BQ provided technical and information support. All authors approved the final version of the manuscript.
Con fl icts of interest:None declared.
Blizzard CA, King AE, Vickers J, Dickson T (2013) Cortical murine neurons lacking the neuro fi lament light chain protein have an attenuated response to injury in vitro. J Neurotrauma 30:1908-1918.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248-254.
Carter J, Gragerov A, Konvicka K, Elder G, Weinstein H, Lazzarini RA (1998) Neurofilament (NF) assembly; divergent characteristics of human and rodent NF-L subunits. J Biol Chem 273:5101-5108.
de Groot DM, Coenen AJ, Verhofstad A, van Herp F, Martens GJ (2006) In vivo induction of glial cell proliferation and axonal outgrowth and myelination by brain-derived neurotrophic factor. Mol Endocrinol 20:2987-2998.
Deng Y, Li B, Liu F, Iqbal K, Grundke-Iqbal I, Brandt R, Gong CX (2008) Regulation between O-GlcNAcylation and phosphorylation of neuro fi lament-M and their dysregulation in Alzheimer disease. FASEB J 22:138-145.
Holmgren A, Bouhy D, Timmerman V (2012) Neurofilament phosphorylation and their proline-directed kinases in health and disease. J Peripher Nerv Syst 17:365-376.
Kuhle J, Gaiottino J, Leppert D, Petzold A, Bestwick JP, Malaspina A, Lu CH, Dobson R, Disanto G, Norgren N, Nissim A, Kappos L, Hurlbert J, Yong VW, Giovannoni G, Casha S (2014) Serum neurofilament light chain is a biomarker of human spinal cord injury severity and outcome. J Neurol Neurosurg Psychiatry doi: 10.1136/jnnp-2013-307454.
Lee S, Sunil N, Shea TB (2011) C-terminal neuro fi lament phosphorylation fosters neuro fi lament-neuro fi lament associations that compete with axonal transport. Cytoskeleton 68:8-17.
Lobsiger CS, Garcia ML, Ward CM, Cleveland DW (2005) Altered axonal architecture by removal of the heavily phosphorylated neuro fi lament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS. Proc Natl Acad Sci U S A 102:10351-10356.
Merlos Rodrigo MA, Zitka O, Krizkova S, Moulick A, Adam V, Kizek R (2014) MALDI-TOF MS as evolving cancer diagnostic tool: a review. J Pharm Biomed Anal 95:245-255.
Minoura I, Hachikubo Y, Yamakita Y, Takazaki H, Ayukawa R, Uchimura S, Muto E (2013) Overexpression, purification, and functional analysis of recombinant human tubulin dimer. FEBS Lett 587:3450-3455.
Peştean C, Taulescu M, Ober C, Cătoi C, Miclăuş V, Oana L, Bodolea C (2013) The effect of chronic toxicity of pethidine on the spinal cord: an experimental model in rabbits. Rom J Morphol Embryol 54:617-622.
Tannu NS, Hemby SE (2006) Two-dimensional fl uorescence difference gel electrophoresis for comparative proteomics pro fi ling. Nat Protocols 1:1732-1742.
Tashiro T, Imai R, Komiya Y (1994) Early effects of beta,beta′-iminodipropionitrile on tubulin solubility and neuro fi lament phosphorylation in the axon. J Neurochem 63:291-300.
Wang HT, Wu MF, Zhan CJ, Ma EY, Yang MG, Yang XY, Li YP (2012) Neuro fi lament proteins in axonal regeneration and neurodegenerative diseases. Neural Regen Res 7:620-626.
Wataya T, Nunomura A, Smith MA, Siedlak SL, Harris PLR, Shimohama S, Szweda LI, Kaminski MA, Avila J, Price DL, Cleveland DW, Sayre LM, Perry G (2002) High molecular weight neurofilament proteins are physiological substrates of adduction by the lipid peroxidation product hydroxynonenal. J Biol Chem 277:4644-4648.
Xiong H, Zheng C, Wang J, Song J, Zhao G, Shen H, Deng Y (2013) The neuroprotection of liraglutide on Alzheimer-like learning and memory impairment by modulating the hyperphosphorylation of tau and neuro fi lament proteins and insulin signaling pathways in mice. J Alzheimers Dis 37:623-635.
Zhu BQ, Yang XY, Gu R, Qiu BT (2010) Difference in proteome Maps between spinal cord ischemia reperfusion injury (IR) and normal rabbit spinal cord. Zhongguo Shiyan Zhenduan Xue 14:487.
Zhu Y, Lee HC, Zhang L (2002) An examination of heme action in gene expression: heme and heme de fi ciency affect the expression of diverse genes in erythroid k562 and neuronal PC12 cells. DNA Cell Biol 21:333-346.
Copyedited by Murphy JS, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
Xiaoyu Yang, M.D., Department of Orthopedics, the Second Hospital of Jilin University, Changchun 130000, Jilin Province, China, yangxiaoyu88@sina.com. Dalin Wang, Department of Orthopedic Surgery, Affiliated Hospital of Beihua University, Jilin, Jilin Province, China, 63193778@163.com.
10.4103/1673-5374.141803
http://www.nrronline.org/
Accepted: 2014-07-25
- 中国神经再生研究(英文版)的其它文章
- Histological assessment in peripheral nerve tissue engineering
- Genetic factors for nerve susceptibility to injuries -lessons from PMP22 de fi ciency
- Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy
- Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury
- Factors affecting directional migration of bone marrow mesenchymal stem cells to the injured spinal cord
- Craniocerebral injury promotes the repair of peripheral nerve injury

