Craniocerebral injury promotes the repair of peripheral nerve injury
Wei Wang, Jun Gao, Lei Na, Hongtao Jiang, Jingfeng Xue, Zhenjun Yang, Pei Wang
1 Department of Hand and Foot Surgery, Af fi liated Hospital of Chengde Medical College, Chengde, Hebei Province, China
2 Department of Postgraduate, Chengde Medical College, Chengde, Hebei Province, China
3 Department of Anatomy, Chengde Medical College, Chengde, Hebei Province, China
Craniocerebral injury promotes the repair of peripheral nerve injury
Wei Wang1, Jun Gao2, Lei Na2, Hongtao Jiang2, Jingfeng Xue3, Zhenjun Yang3, Pei Wang1
1 Department of Hand and Foot Surgery, Af fi liated Hospital of Chengde Medical College, Chengde, Hebei Province, China
2 Department of Postgraduate, Chengde Medical College, Chengde, Hebei Province, China
3 Department of Anatomy, Chengde Medical College, Chengde, Hebei Province, China
The increase in neurotrophic factors after craniocerebral injury has been shown to promote fracture healing. Moreover, neurotrophic factors play a key role in the regeneration and repair of peripheral nerve. However, whether craniocerebral injury alters the repair of peripheral nerve injuries remains poorly understood. Rat injury models were established by transecting the left sciatic nerve and using a free-fall device to induce craniocerebral injury. Compared with sciatic nerve injury alone after 6-12 weeks, rats with combined sciatic and craniocerebral injuries showed decreased sciatic functional index, increased recovery of gastrocnemius muscle wet weight, recovery of sciatic nerve ganglia and corresponding spinal cord segment neuron morphologies, and increased numbers of horseradish peroxidase-labeled cells. These results indicate that craniocerebral injury promotes the repair of peripheral nerve injury.
nerve regeneration; craniocerebral injury; peripheral nerve; sciatic nerve; sciatic nerve injury; nerve repair; horseradish peroxidase tracer technique; neural regeneration
Funding:This study was supported by a grant from Hebei Provincial Science and Technology Department in China, No. 142777105D, 13277772D.
Wang W, Gao J, Na L, Jiang HT, Xue JF, Yang ZJ, Wang P. Craniocerebral injury promotes the repair of peripheral nerve injury. Neural Regen Res. 2014;9(18):1703-1708.
Introduction
Traumatic brain injury often occurs in combination with limb fractures and peripheral nerve injury. The promotion of fracture healing by traumatic brain injury has been reported by many studies (Spencer, 1987; Morley et al., 2005). Gibson (1960) first reported that a large number of bone calluses formed in the fracture site of femoral fracture patients that also had brain injuries, which has been verified by many animal and clinical experiments (Perkins and Skirving, 1987; Spencer, 1987). After brain injury, damage to the blood-brain barrier increases its permeability, allowing several osteogenic factors to enter the systemic circulation. In this manner, the expression of such factors in serum was markedly increased, promoting callus formation and accelerating fracture healing (Khare et al., 1995; Liu et al., 2012; Yang et al., 2012b). Cytokines, neuropeptides, neurotrophic factors, humoral factors, and mechanical factors can affect fracture healing after craniocerebral injury (Shen et al., 2012; Yang et al., 2012a; Liu et al., 2013b; Yan et al., 2013; Zhang et al., 2013; Zhao et al., 2014). Numerous studies have demonstrated that serum nerve growth factor, brain-derived neurotrophic factor, and basic fi broblast growth factor expression is higher in patients with fracture and craniocerebral injury than in patients with fracture alone (Wildburger et al., 1994; Yang and Dong, 2012; Zhuang and Li, 2013). Neurotrophic factors play important roles in the repair of peripheral nerve injury (Hong et al., 1999; Liu et al., 2013a; Wang et al., 2013; Yu et al., 2014). The aim of the present study was to determine whether changes in the body, such as neurotrophic factors, caused by craniocerebral injury promote the repair of peripheral nerve.
Materials and Methods
Experimental animals
A total of 80 male specific-pathogen-free Sprague-Dawley rats aged 8 weeks and weighing 200-220 g were purchased from Vital River Laboratories, Beijing, China (license No. SCXK (Jing) 2012-0001). They were housed in a 12-hour light/dark cycle at 23 ± 2°C. The protocols used conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised 1996), and the protocol was approved by the Institutional Animal Care Committee of Chengdu Medical University in China. The rats were equally and randomly divided into injury and control groups. The injury group received a craniocerebral injury and sciatic nerve transection, while the control group received only the sciatic nerve transection.
Models of craniocerebral injury combined with sciatic nerve transection
Injury group: Using the classical Feeney method (Feeney etal., 1981), the rats were intraperitoneally anesthetized with 10% chloral hydrate (0.35 g/kg), restrained, and sterilely prepared. A sagittal incision was made on the scalp to expose the right parietal bone. A bone window of 5 mm diameter was drilled 1.5 mm posterior to the coronal line and 2.5 mm lateral to the median line using a dental drill. The cerebral dura mater was kept intact. A 20 g falling hammer was dropped freely from a height of 30 cm along the peripheral catheter, striking the bar to make a moderate contusion of right parietal bone. The bone window was sealed with bone wax, and the scalp was sutured. After sterilization, an incision was made in the left buttock to expose the sciatic nerve. The sciatic nerve was transected 1 cm below the lower hole of the left piriformis of the rats, and then sutured with 9-0 nontraumatic thread using an epicardial suture technique under a microscope (LEL-6A; Zhongtian Optical Instrument Co., Ltd., Zhenjiang, Jiangsu Province, China). Finally, the skin was sutured closed (Cheng and Li, 2006).
Control group: After anesthesia and restraint, the left sciatic nerve was transected and sutured as detailed above. The rats in both groups were housed in individual cages.
Measurement of sciatic functional index
Ten rats were collected from both groups at 4, 6, 8, and 12 weeks after injury. Custom-made walking dark chambers were used, as in a previous study (Schiaveto de Souza et al., 2004), to measure footprints. Three parameters were measured on both the experimental side (E) and contralateral normal side (N). (1) Footprint length (PL): from heel to toe; (2) toe width (TS): from the fi rst toe to the fi fth toe; (3) toe spacing (IT): from the second toe to the fourth toe. From these parameters, the sciatic functional index was calculated as - 38.3 × (EPL - NPL)/NPL + 109.5 × (ETS - NTS)/NTS + 13.3 × (EIT - NIT)/NIT - 8.8. The sciatic functional index expressed as absolute value ranged from: 0, representing normal, to 100, representing complete nerve damage and loss of function.
Measurement of gastrocnemius muscle wet weight
At 4, 6, 8, and 12 weeks after injury, 10 rats were selected from both groups. After anesthesia with intraperitoneal injection of chloral hydrate, the entire gastrocnemius muscle was harvested from the medial and external femoral condyles to the calcaneal tuberosity. The wet weight (g) of the gastrocnemius muscle was assessed using an electronic balance (ESJ200-4; Dragon Electronics Co., Ltd., Shenyang, Liaoning Province, China). The recovery of gastrocnemius muscle wet weight (%) was calculated as the wet weight on the experimental side (g) divided by the wet weight on the normal side (g) ×100%.
Histopathological observation of nervous tissues using hematoxylin-eosin staining
Sciatic nerve stumps were selected at 4, 6, 8, and 12 weeks after injury. All samples were fi xed in 10% neutral formalin for 24 hours, dehydrated through a graded alcohol series, embedded in paraf fi n, and longitudinally sliced into 5 μm-thick sections. These sections were dewaxed with xylene, hydrated through a graded alcohol series, stained with hematoxylin, differentiated with ethanol hydrochloride, immersed in running water, dipped in eosin, dehydrated, permeabilized, and mounted. Nerve fi ber morphology was observed with a light microscope (BH-2; Olympus, Tokyo, Japan).
Repair of sciatic nerve pathway observed with a horseradish peroxidase tracer
At 4, 6, 8 and 12 weeks after injury, 10 rats were selected from each group. After anesthesia as above, the nerve was slightly clipped 0.5 cm from the left sciatic nerve stump, and 30% horseradish peroxidase solution (Sigma, St. Louis, MO, USA) was infused. After 72 hours, the chest was opened under deep anesthesia, and the ascending aorta of the left ventricle was cannulated. The blood vessels were rinsed with 200 mL of warm physiological saline. Perfusion and fi xation were performed with 400 mL of 2% paraformaldehyde and 2% glutaral prepared in 0.1 mol/L phosphate buffer. The ganglia of the sciatic nerve and corresponding segments at the T4-5spinal levels were serially sliced into 50 μm-thick sections in the cross-sectional plane with a vibratome. These sections were washed with 0.1 mol/L PBS (pH 7.4), and treated with benzidine dihydrochloride. After exposure to 0.3% H2O2, sections were immersed in stabilizing buffer, washed with distilled water, and counterstained with neutral red (Mesulam and Rosene, 1977). The number of cell bodies containing blue-stained particles in the ganglia and motor neurons of the spinal anterior horn was observed with a light microscope (Olympus).
Statistical analysis
The data were analyzed using SPSS 17.0 software, and were expressed as mean ± SD. Intergroup comparisons were made using two-samplet-tests. Values ofP< 0.05 were considered statistically signi fi cant.
Results
Effects of craniocerebral injury on the gross state of rats with peripheral nerve injury
All animals survived the surgeries.ree weeks later, 30 rats in the injury group showed red swelling of their left lower extremity. In the control group, 32 rats showed ulcers and movement disorder. At 6 weeks, swelling of the left lower limb subsided, and the ulcers began to diminish in the injury group. In contrast, the swelling was still present in the control group. At 8 weeks, the symptoms started to improve in rats from the control group, and the symptoms in the injury group were noticeably improved. At 12 weeks, the symptoms in rats of both groups were further improved.
Effects of craniocerebral injury on the sciatic functional index of rats with peripheral nerve injury
The dark chamber walking results demonstrated that at 4 weeks after injury, the sciatic functional index was similar between the injury and control groups (P> 0.05). After 4 weeks, the sciatic functional index began to decrease in theinjury group. With time, the rate of decrease slowed.e sciatic functional index decreased aer 4 weeks in the control group. At 8 and 12 weeks, the sciatic functional index was lower in the injury group than in the control group (P< 0.01; Table 1).
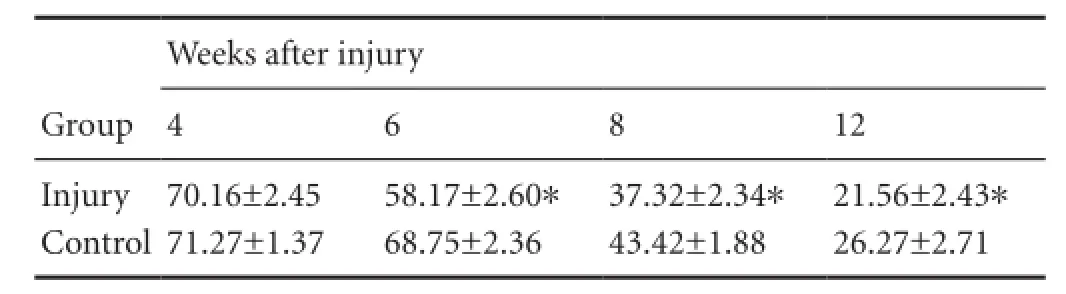
Table 1 Effects of craniocerebral injury on sciatic functional index in rats with peripheral nerve injury
Effects of craniocerebral injury on the gastrocnemius muscle wet weight of rats with peripheral nerve injury
Effects of craniocerebral injury on the pathological changes in sciatic nerve of rats with peripheral nerve injury
Hematoxylin-eosin staining demonstrated no signi fi cant differences between the injury and control groups at 4 weeks. No nerve fi bers traversed the nerve stump, which was irregular and had no nerve fi bers integrated. Many nerve fi bers were observed in the proximal end in the injury group, and a large number of vacuoles and necrotic cells were seen in the control group. At 6 weeks, a few sparse nerve fi bers traversed the nerve stump, and these nerve fibers were not uniform, but irregularly arranged in the injury group. Nerve fibers did not traverse the nerve stump, and vacuolar degeneration was apparent in many cells in the control group. At 8 weeks, many nerve fibers traversed the nerve stump, and these nerve fi bers were uniform and regular, though still thin, in the injury group. The nerve fibers that traversed the nerve stump were not uniform, but were irregularly arranged in the control group. At 12 weeks, numerous nerve fi bers of a large diameter traversed the nerve stump and were arranged regularly in the injury group, appearing similar to normal fi bers. In the control group, many uniform nerve fi bers traversed the nerve stump, showing a typical wavy arrangement (Figure 1).
Effects of craniocerebral injury on the repair of the sciatic nerve pathway of rats with peripheral nerve injury
At 4 weeks, no dark blue-stained neuronal bodies were de-tected by light microscopy (200 ×), but numerous swollen cells were visible in the ganglion and spinal anterior horn in both the injury and control groups. At 6 weeks, labeled neuronal bodies were observed in the ganglion of the injury group, but not in the control group. At 8 weeks, labeled cells were apparent in the ganglion and spinal anterior horn in the injury group, averaging 12-15 cells/ fi eld. A few labeled cells were detectable in the control group, averaging 2-5 cells/ fi eld. At 12 weeks, labeled cells were visible in both the injury and control groups (Figure 2).
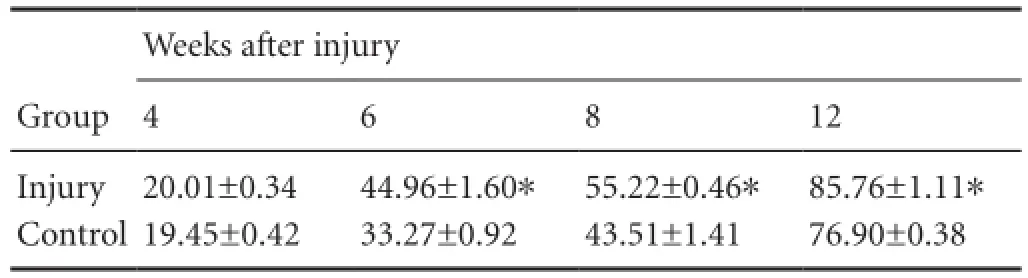
Table 2 Effects of craniocerebral injury on the recovery of gastrocnemius muscle wet weight (%) of rats with peripheral nerve injury
Discussion
After peripheral nerve injury that includes transection of axons, Wallerian degeneration occurs, presenting swollen neuronal bodies, nuclear deviation, and chromatolysis. For example, neuronal bodies died in spinal ganglion and spinal cord of cats following sciatic nerve transection (Risling et al., 1983; Arvidsson et al., 1986). In the present study, a large number of necrotic and degenerated nerve fibers were observed during the repair phase at 4 weeks after sciatic nerve transection in rats in both the injury and control groups. Moreover, neuronal cells were also swollen, degenerated, and apoptotic in the ganglion and corresponding segments of the spinal cord, which confirmed this type of pathological changes. With time after injury, the neurons became regular and function was recovered in the sciatic nerve ganglion and corresponding segments of spinal cord in both groups, which indicates that the key to neurological recovery is the survival and regenerative capacity of damaged neurons (Gillardon et al., 1996; Pan et al., 2009; Dong et al., 2011). After peripheral nerve injury, the death of neuronal bodies was associated with animal age, the axonal injury site, the nature of the damage, and the neuronal type (Yegiyants et al., 2010; Wang et al., 2011). Here, at 6, 8, and 12 weeks, the quantity and quality of neurons in the ganglion and anterior horn of the spinal cord were better in the injury group than in the control group. Whether the craniocerebral injury reduced the death of neurons deserves further investigation.

Figure 1 Effects of craniocerebral injury on the pathological changes in sciatic nerve of rats with peripheral nerve injury (hematoxylin-eosin staining, × 100).
The conditions for successful repair and regeneration of injured peripheral nerve include the survival and restoration of injured neuronal cell bodies, as well as sprouting and elongation of the proximal axons (English et al., 2011). Mature myelinated nerve fi bers in regenerated nerves indicate an effective neural regeneration (Xu et al., 2003). In the present study, at 6, 8, and 12 weeks, nerve fi bers were observed in the nerve stump, clearly indicating that the injured peripheral nerve here could be repaired and regenerated. In addition, nerve fi bers traversed the nerve stump earlier in the injury group (with craniocerebral injury) than in the control group (without craniocerebral injury). Within the same time period, more regularly arranged nerve fi bers traversing the nerve stump were found in the injury group compared with the control group, suggesting that craniocerebral injury contributed to the repair of the sciatic nerve injury to some extent. Moreover, the ultimate aim of peripheral nerve repair is to restore the function of a target muscle. The evaluation of the reinnervation rate of a target muscle can also be used to assess nerve repair and regeneration (Liang et al., 2007). In the present study, the recovery of the gastrocnemius muscle was signi fi cantly higher in the injury group than in the control group at 6, 8, and 12 weeks, which suggested that the craniocerebral injury promoted the repair of the peripheral nerve injury. Recently, a horseradish peroxidase marking method has been extensively used to trace nerves and to study neural regeneration (Schiaveto de Souza et al., 2004). In the present study, at 4 weeks, Wallerian degeneration was detected, and the sciatic functional index was similar between the two groups. The sciatic functional index decreased at later time points, indicating that regenerated axons had traversed the stumps, reinnervated target muscles, partially restored muscle force, and coordinated the function among muscles. At 8 and 12 weeks, the sciatic functional index was clearly lower in the injury group than in the control group. To some extent, the craniocerebral injury promoted the growth of regenerated axons in large numbers, contributing to the formation and maturation of the neuromuscular junction.
The horseradish peroxidase retrograde tracer technique has been widely used to trace nerves. Horseradish peroxidase can be absorbed and transported by nerve endings with various functions. It also accumulates in nerve cell bodies, and its transport is mainly associated with microtubules in nerve fibers (Seckel et al., 1984; Mearow et al., 1994). Therefore, nerve fi bers that have lost their continuity cannot transport horseradish peroxidase from the distal to proximal end or accumulate it in their cell bodies. Horseradish peroxidase-labeled cells in the spinal ganglion and spinal cord can be used to identify whether regenerated fi bers traversed the suture site after end-to-end suture. In this study, at 4 weeks after surgery, labeled neuronal cells were not detected in the rat ganglion or spinal cord in either the injury or control groups, indicating that the regenerated fibers did not traverse the nerve stumps. At 6 weeks, labeled neuronal cells were detected in the rat spinal ganglion in the injury group, but not in the control group, indicating that the regenerated fi bers of rats with craniocerebral injury traversed the nerve stumps earlier than those in the control rats. After craniocerebral injury, several factors can promote the repair of injured peripheral nerves. At 8 and 12 weeks after surgery, a large number of labeled cells were visible in the spinal ganglion and spinal cord in both groups, but the number was obviously higher in the injury group than in the control group. These results suggest that changes in the bodies of therats after craniocerebral injury protected the neuronal cells, contributed to the better restoration of neuronal cells, and played a role in the repair of the peripheral nerve injury.
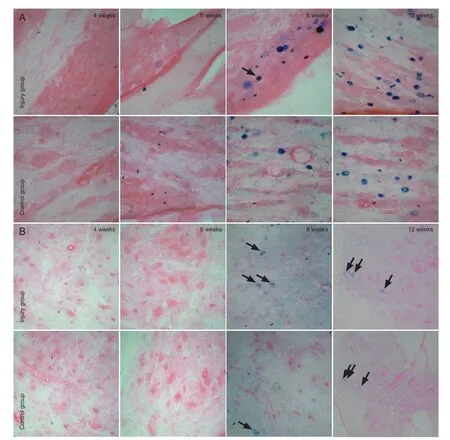
Figure 2 Horseradish peroxidase-labeled neuronal cells in the sciatic nerve ganglion (A) and segments of the spinal cord (B) in rats with peripheral nerve injury (× 200).
In summary, the regeneration of broken sciatic nerves occurred faster in rats with craniocerebral injuries than in control rats without craniocerebral injuries, indicating that the craniocerebral injury promoted the repair of the peripheral nerve injury. However, the precise mechanisms remain poorly understood and deserve further investigation.
Author contributions:This study was designed by Wang W and Wang P, and performed by Wang W, Gao J, Na L, JiangHT and Wang P. Xue JF and Yang ZJ evaluated this study, and provided data and technical support. Wang W wrote the manuscript. Wang P was in charge of manuscript authorization. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Arvidsson J, Ygge J, Grant G (1986) Cell loss in lumbar dorsal root ganglia and transganglionic degeneration aer sciatic nerve resection in the rat. Brain Res 373:15-21.
Cheng SA, Li ZY (2006) The expression of GDNF in injured sciatic nerve of rats. Zhejiang Yixue 28:353-355.
Dong YZ, Liang QD, Diuan YZ, Shi XG (2011)e study of protective e ff ect of olfactory ensheathing cells on motoneurons in ventral horn of the spinal cord. Chongqing Yixue 40:2096-2098.
English AW, Cucoranu D, Mulligan A, Rodriguez JA, Sabatier MJ (2011) Neurotrophin-4/5 is implicated in the enhancement of axon regeneration produced by treadmill training following peripheral nerve injury. Eur J Neurosci 33:2265-2271.
Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG (1981) Responses to cortical injury: I. Methodology and local e ff ects of contusions in the rat. Brain Res 211:67-77.
Gibson JM (1960) Multiple injuries: management of the patient with a fractured femur and a head injury. J Bone Joint Surg Br 42:425.
Gillardon F, Klimaschewski L, Wickert H, Krajewski S, Reed JC, Zimmermann M (1996) Expression pattern of candidate cell death e ff ector proteins Bax, Bcl-2, Bcl-X, and c-Jun in sensory and motor neurons following sciatic nerve transection in the rat. Brain Res 739:244-250.
Hong A, Li XK, Fu ZG, Xu L, Lin J (1999) Effect of basic fibroblast growth factor on repairing transected sciatic nerve in rats. Zhongguo Xiufu Chongjian Waike Zazhi 13:287-290.
Khare GN, Gautam VK, Gupta LN, Gupta AK (1995) A new hypothesis for faster healing of fractures in head injured patients. Indian J Med Sci 49:281-284.
Liang AL, Jiang DM, An H (2007) Effects of glial cell line-derived neurotrophic factor gene-activated matrix on repair of sciatic nerve defect of rats. Disan Junyi Daxue Xuebao 29:717-720.
Liu GH, Zhang L, Liang CY (2012) Brain injury with fracture can accelerate fracture healing and heterotopic ossi fi cation. Zhongguo Zuzhi Gongcheng Yanjiu 16:8721-8726.
Liu HW, Wen WS, Hu M, Bi WT, Chen LJ, Liu SX, Chen P, Tan XY (2013a) Chitosan conduits combined with nerve growth factor microspheres repair facial nerve defects. Neural Regen Res 8:3139-3147.
Liu X, Zhou C, Li Y, Ji Y, Xu G, Wang X, Yan J (2013b) SDF-1 promotes endochondral bone repair during fracture healing at the traumatic brain injury condition. PLoS One 8:e54077.
Mearow KM, Kril Y, Gloster A, Diamond J (1994) Expression of NGF receptor and GAP-43 mRNA in DRG neurons during collateral sprouting and regeneration of dorsal cutaneous nerves. J Neurobiol 25:127-142.
Mesulam MM, Rosene DL (1977) Di ff erential sensitivity between blue and brown reaction procedures for HRP neurohistochemistry. Neurosci Lett 5:7-14.
Morley J, Marsh S, Drakoulakis E, Pape HC, Giannoudis PV (2005) Does traumatic brain injury result in accelerated fracture healing? Injury 36:363-368.
Pan SP, Liu Q, Wu D (2009) Protective e ff ect of glial cell line-derived neurotrophic factor infused into the tube setted into cavitas subarachnoidealis on spinal front corner motor neurons. Zhongguo Gushang 22:122-124.
Perkins R, Skirving AP (1987) Callus formation and the rate of healing of femoral fractures in patients with head injuries. J Bone Joint Surg Br 69:521-524.
Risling M, Aldskogius H, Hildebrand C, Remahl S (1983) Effects of sciatic nerve resection on L7 spinal roots and dorsal root ganglia in adult cats. Exp Neurol 82:568-580.
Schiaveto de Souza A, da Silva CA, Del Bel EA (2004) Methodological evaluation to analyze functional recovery aer sciatic nerve injury. J Neurotrauma 21:627-635.
Seckel BR, Chiu TH, Nyilas E, Sidman RL (1984) Nerve regeneration through synthetic biodegradable nerve guides: regulation by the target organ. Plast Reconstr Surg 74:173-181.
Shen F, Zeng Z, Pan HT (2012) Role of transforming growth factor-β1 during fracture healing in patients with fracture and cerebral trauma. Zhongguo Gu yu Guanjie Sunshang Zazhi 601-604.
Spencer RF (1987) The effect of head injury on fracture healing. A quantitative assessment. J Bone Joint Surg Br 69:525-528.
Wang G, Fan SW, Li Q, Shen TG (2013) Clincal e ff ects of nerve growth factor gradient release system on treatment of peripheral nerve injuries. Zhonghua Xianwei Waike Zazhi 36:558-562.
Wang W, Yuan XH, Wang ZL, Liu NL, Zhang LX, Li LG (2011) E ff ects of magnetic stimulation on nerve conduction velocity and the expression of GAP-43 aer sciatic nerve injury in rats. Shandong Yiyao 51:20-22.
Wildburger R, Zarkovic N, Egger G, Petek W, Zarkovic K, Hofer HP (1994) Basic fi broblast growth factor (BFGF) immunoreactivity as a possible link between head injury and impaired bone fracture healing. Bone Miner 27:183-192.
Xu X, Yee WC, Hwang PYK, Yu H, Wan ACA, Gao S, Boon KL, Mao HQ, Leong KW, Wang S (2003) Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials 24:2405-2412.
Yan H, Zhang HW, Fu P, Liu BL, Jin WZ, Duan SB, Xue J, Liu K, Sun ZM, Zeng XW (2013) Leptin’s e ff ect on accelerated fracture healing aer traumatic brain injury. Neurol Res 35:537-544.
Yang HL, Dong JB (2012) The expression and clinical significance of serotonin and NGF in the serum of traumatic brain injury combined fracture patients. Zhongguo Xiandai Yixue Zazhi 22:44-47.
Yang S, Ma Y, Liu Y, Que H, Zhu C, Liu S (2012a) Arachidonic acid: a bridge between traumatic brain injury and fracture healing. J Neurotrauma 29:2696-2705.
Yang TY, Wang TC, Tsai YH, Huang KC (2012b)e e ff ects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral sha. J Bone Joint Surg Br 94:227-230.
Yegiyants S, Dayicioglu D, Kardashian G, Panthaki ZJ (2010) Traumatic peripheral nerve injury: a wartime review. J Craniofac Surg 21:998-1001.
Yu H, Liu J, Ma J, Xiang L (2014) Local delivery of controlled released nerve growth factor promotes sciatic nerve regeneration aer crush injury. Neurosci Lett 566:177-181.
Zhang L, Zhang L, Mao Z, Tang P (2013) Semaphoring 3A: an association between traumatic brain injury and enhanced osteogenesis. Med Hypotheses 81:713-714.
Zhao JP, Huang CY, Li QF (2014) The study of the expression of HIF-1 in osteotylus of brain injury associated with fracture. Yixue Xinxi 27:154-155.
Zhuang YF, Li J (2013) Serum EGF and NGF levels of patients with brain injury and limb fracture. Asian Pac J Trop Med 6:383-386.
Copyedited by McCarty W, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
Pei Wang, Department of Hand and Foot Surgery, Affiliated Hospital of Chengde Medical College, Chengde 067000, Hebei Province, China, cdgkwp@sina.com.
10.4103/1673-5374.141807
http://www.nrronline.org/
Accepted: 2014-06-19
- 中国神经再生研究(英文版)的其它文章
- Histological assessment in peripheral nerve tissue engineering
- Genetic factors for nerve susceptibility to injuries -lessons from PMP22 de fi ciency
- Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy
- Oxidative phosphorylated neuro fi lament protein M protects spinal cord against ischemia/reperfusion injury
- Ginsenoside Rd inhibits apoptosis following spinal cord ischemia/reperfusion injury
- Factors affecting directional migration of bone marrow mesenchymal stem cells to the injured spinal cord

