Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
Yang Wang, Zhouyan Feng, Jing Wang, Xiaojing Zheng
Key Laboratory of Biomedical Engineering of Education Ministry, College of Biomedical Engineering and Instrumentation Science, Zhejiang University, Hangzhou, Zhejiang Province, China
Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
Yang Wang, Zhouyan Feng, Jing Wang, Xiaojing Zheng
Key Laboratory of Biomedical Engineering of Education Ministry, College of Biomedical Engineering and Instrumentation Science, Zhejiang University, Hangzhou, Zhejiang Province, China
Zhouyan Feng, Ph.D., Key Laboratory
of Biomedical Engineering of Education
Ministry, College of Biomedical
Engineering and Instrumentation Science, Zhejiang University, Hangzhou 310027, Zhejiang Province, China,
fengzhouyan@139.com.
nerve regeneration; somatosensory stimulation; tail clamping; hippocampal CA1 region; local field potential; unit spike; population spike; excitability; 973 Program; neural regeneration
Funding: This work was supported by Major State Basic Research Development Program of China (973 Program), No. 2011CB504400.
Wang Y, Feng ZY, Wang J, Zheng XJ. Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats. Neural Regen Res. 2014;9(11):1138-1144.
Introduction
The hippocampal region of the brain is known to play important roles in learning and memory formation. Understanding the behavior of individual neurons in the hippocampus is crucial for elucidating the mechanisms of memory formation. Currently, it is not clear how hippocampal neurons respond to inputs from the sensory nervous system (Pereira et al., 2007). Previous investigations have shown that sensory stimulation, such as strokes on the foot or whiskers, evoke different patterns of action potential firing in hippocampal neurons, which can be detected as a change in multiple unit activity (Miller and Groves, 1977; Vinogradova et al., 1993; Pereira et al., 2007). In addition, in response to somatosensory stimulation, the fi ring of pyramidal cells in the CA1 region decreases, while the firing of interneurons changes intricately-increasing, decreasing or without significant change (Miller and Groves, 1977; Vinogradova et al., 1993; Bellistri et al., 2013). However, the mechanisms responsible for the activity change in pyramidal cells (i.e., the principal cells in the hippocampus) remain unclear. Considering that these cells are modulated by local inhibitory circuits comprised of interneurons (Zheng and Khanna, 2001; Bellistri et al., 2013), the decrease in pyramidal cell fi ring could be caused by an increase in interneuron-mediated inhibition, or by a decrease in pyramidal cell excitability. In addition, a decrease in afferent input could also affect pyramidal cell activity (Bellistri et al., 2013).
To investigate how pyramidal cells and interneurons respond to somatosensory input in the hippocampal CA1 region, we recorded and analyzed changes in local field potentials and fi ring rates of individual neurons during tail clamping in urethane-anesthetized rats. We also investigated the mechanisms underlying the neuronal responses.
The urethane anesthetized preparation can avoid the infl uence of animal’s behavior and other unnecessary sensory input (Deadwyler et al., 1981; Itskov et al., 2011) without signi fi cant anesthetic effects on somatosensory inputs, because the urethane has minimum inhibition on sensory-evoked responses in nervous systems (Sceniak and Maciver, 2006).
Materials and Methods
Electrode placement and signal recording
A total of 12 clean, healthy, adult, male, Sprague-Dawley rats weighing 250-350 g were provided by the Experimental Animal Center, Zhejiang Academy of Medical Science, China. All procedures used in this study were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Health, China), and the protocol was approved by the Institutional Animal Care Committee of Zhejiang University in China. The rats were intraperitoneal-ly anesthetized with urethane 1.25-1.50 g/kg, and placed in a stereotaxic apparatus (Stoelting Co., Wood Dale, IL, USA). Part of the skull was removed for placement of electrodes. The recording electrode was a 16-channel microelectrode array (NeuroNexus Technologies, Ann Arbor, MI, USA) that was inserted into the hippocampal CA1 region (anteroposterior, -3.0 mm; mediolateral, 2.6 mm; dorsoventral, 2.5 mm) (George and Charles, 2007) to make extracellular recordings of local fi eld potentials and to record unit spikes. The stimulating electrode was a bundled pair of polyimide insulated tungsten electrodes (A-M Systems Inc., Carlsborg, WA, USA) with a vertical tip separation of about 0.5 mm, and was inserted into the Schaffer collaterals (anteroposterior, -2.0 mm; mediolateral, 2.3 mm; dorsoventral, 2.8 mm) for orthodromic stimulation of CA1 neurons. Two stainless steel screws were fi xed in the nasal bone and served as reference and ground. The accuracy of electrode placement was confi rmed by the unique waveforms of the orthodromic-evoked potentials in the CA1 region recorded by the electrode array (Kloosterman et al., 2001). Saline was poured over the exposed cortex to maintain moisture.
Stimulus pulses with constant currents were produced by a Model 2300 Stimulus Isolator (A-M Systems Inc.), with a duration of 0.1 ms and a current intensity in the range of 0.25-0.35 mA, that induced orthodromic-evoked population spikes of about 80% maximal amplitude in the CA1 stratum pyramidale. The population spike amplitude was measured as the average of the two potential differences of the negative spike peak to the preceding and following positive peaks.
Signals were first amplified 100-fold by a 16-channel ampli fi er (Model 3600, A-M Systems Inc.) with a fi lter frequency range of 0.3-5,000 Hz. Signals were then sampled at 20,000 Hz with a data-acquisition system PowerLab ML880 (AD Instruments Inc., Castle Hill, NSW, Australia) and were stored onto hard disk for of fl ine analysis (Feng et al., 2013).
Signal processing and spike sorting
Using the LabChart software in the PowerLab ML880 suite, the local fi eld potentials were extracted from the raw recording signals by a 0.5-80 Hz digital band-pass fi lter, then the power spectrum of a 30-second long local field potential signal was estimated using Welch’s method (Welch, 1967), i.e., the spectrum was calculated by fast Fourier transformation with a length of 217sampling data (about 6.55 seconds) in successive 50% overlapping Hanning windows. The frequency resolution of the estimated spectrum was about 0.15 Hz. Then, the power percentages of local fi eld potentials in the following sub-frequency bands were calculated: delta (0.5-2 Hz), theta (2-7 Hz), alpha (7-13 Hz), beta (13-30 Hz) and gamma (30-80 Hz) (Buzsáki and Draguhn, 2004).
The digital high-pass fi lter provided in the LabChart software with a cut-off frequency of 500 Hz was used to extract the multiple unit activity signals from the raw recordings from the CA1 stratum pyramidale. Unit spikes were then detected using a threshold method (Lewicki, 1998). The threshold values were set as ± 5-8 times the standard deviation of the multiple unit activity signal to minimize error. Signals with amplitudes larger than the threshold were collected as unit waveforms with a duration of 4 ms (2 ms pre-threshold and 2 ms post-threshold). The feature vectors (i.e., principal components) of the unit spike waveforms were extracted with a Matlab program, MClust (http://redishlab.neuroscience.umn.edu/MClust/MClust.html). Then, spike sorting was performed with an open-source automatic clustering software, KlustaKwik (Rutgers University, New Brunswick, NJ, USA) (Harris et al., 2000), which is based on the principal component analysis method.
To distinguish the unit spikes of pyramidal cells from that of interneurons, we calculated the mean waveform widths of the sorted unit spikes. Because the trough to post-peak width for the spike waveforms of a pyramidal cell and an interneuron are 0.86 ± 0.17 ms and 0.43 ± 0.27 ms, respectively (Barthó et al., 2004), a width of 0.7 ms was used to distinguish pyramidal neurons from interneurons. In addition, autocorrelograms of the inter-spike intervals of the sorted unit spikes were used to con fi rm the judgment of neuronal type. CA1 pyramidal cells usually fi re spike bursts of 3-5 or more action potentials with very short inter-spike intervals (Ranck, 1973), yielding two sharp peaks at the short intervals near the center of the autocorrelogram, while autocorrelograms for interneurons appear smooth without sharp peaks (Csicsvari et al., 1999; Barthó et al., 2004).
The raster plots and the peristimulus time histogram of sorted unit spikes were used to calculate the change in fi ring rate of single neurons 30 seconds before and 30 seconds after the onset of somatosensory stimulation (tail clamping). In the peristimulus time histogram, the bin width of the horizontal axis was set at 1 second. Plots of peristimulus time histogram were also used to show the change in fi ring rate of multiple unit spikes.
Somatosensory stimulation
Somatosensory stimulation was applied by clamping the tail for 3 minutes using a crocodile clip with a length of 35 mm (Sute Inc., Shanghai, China) (Bermudez Contreras et al., 2013).
Statistical analysis
All data were expressed as mean ± SD, and analyzed using SPSS for Windows (SPSS, Chicago, IL, USA). Paired t-test was used to evaluate differences in fi ring rates of pyramidal cells and interneurons before and during somatosensory stimulation. One-way analysis of variance and repeated analysis of variance with post hoc Bonferroni tests were used to compare differences in the power spectra of local fi eld potentials and the amplitudes of orthodromic-evoked population spikes before, during and after somatosensory stimulation.
Results
Changes in local fi eld potentials and multiple unit activity under somatosensory stimulation
The local fi eld potential signal in the stratum pyramidale of the CA1 region was examined from one channel of the recording electrode array (Figure 1A). The waveforms of localfi eld potentials changed immediately following the onset of tail clamping with a clip and were maintained for 3 minutes until the removal of the clip. Before tail stimulation, the local fi eld potentials exhibited large-amplitude slow activity with a main power in the delta band. However, during the tail clamping period, local fi eld potentials changed into a theta rhythm-dominated waveform. After removing the clip, the local field potentials recovered quickly to the original slow rhythm (Figure 1B).
The changes in local fi eld potentials were quanti fi ed using power spectrums for 30-second episodes of local fi eld potential signals before and following the onset of tail clamping, as well as after the end of tail clamping. As shown inFigure 1C,during tail clamping, the power percentage of the delta rhythm signi fi cantly decreased by 54.5 ± 13.2% (one-way analysis of variance, F > 283.8, P < 0.001; with post hoc Bonferroni test, P < 0.001; n = 12), while that of the theta rhythm significantly increased by 25.5 ± 9.0% (one-way analysis of variance, F > 24.7, P < 0.001; with post hoc Bonferroni test, P < 0.001; n = 12). The power percentages of the other three bands with higher frequencies were also increased signi fi cantly during tail clamping, but their powers were far less than the theta band.
The signi fi cant changes in local fi eld potential induced by somatosensory stimulation might be associated with changes in unit spike activity. Therefore, the peristimulus time histogram plots of multiple unit activity were used to show the differences in fi ring rates of the multiple unit spikes in the period 30 seconds before and 30 seconds following the onset of tail clamping. However, there were no consistent multiple unit activity changes (n = 12). Some of the peristimulus time histogram plots showed a decrease in spike firing rate following the onset of tail clamping (Figure 1D), while others did not show obvious changes (Figure 1E). Our investigation of single unit activity suggested that the inconsistent changes in multiple unit activity could be caused by the activity of different types of neurons, not by variations in tail clamping or other factors.
Studies have shown that an increase in theta rhythm in the local field potential in the hippocampal CA1 region is usually accompanied by an increase in firing of interneurons (Buzsáki et al., 1986; Toth et al., 1993; Buzsáki, 2002; Bienvenu et al., 2012). In addition, it has been shown that somatosensory stimulation decreases the fi ring of pyramidal cells in the CA1 region (Bellistri et al., 2013). Therefore, we next examined whether tail clamping could induce contrasting changes in pyramidal cells and interneurons in the CA1 region, which could account for the inconsistent changes in multiple unit activity.
Different responses of pyramidal cells and interneurons induced by somatosensory stimulation
To study the responses of pyramidal cells and interneurons to somatosensory stimulation, we sorted the multiple unit activity signals into single unit spikes, and then distinguished the two types of neurons based on the widths of spike waveforms and the autocorrelogram of inter-spike-intervals. As shown inFigure 2A, the superimposed spike waveforms of the pyramidal neurons have a longer trough to post-peak interval than the spike waveforms of interneurons. The autocorrelogram of pyramidal neurons usually contains obvious peaks at very short inter-spike intervals, while that of interneurons does not have this feature. In this study, 10 pyramidal cells and 17 interneurons with an original fi ring rate greater than 2 spikes/s were obtained from 12 rats. The raster plots of these neurons showed that under tail clamping, the spike fi ring rates of pyramidal cells decreased, while that of interneurons increased (Figure 2B). The average peristimulus time histogram plots of the two types of neurons also clearly showed these opposite changes induced by somatosensory stimulation (Figure 2C). The fi ring rates of the neurons in the two 30-second periods before and after the onset of tail clamping were calculated. The fi ring rates of pyramidal cells decreased signi fi cantly from 3.0 ± 1.6 to 1.1 ± 0.9 spike/s (paired t-test, P < 0.001, n = 10), and that of interneurons increased signi fi cantly from 3.3 ± 1.3 to 7.1 ± 7.7 spike/s (paired t-test, P < 0.05, n = 17;Figure 3). Opposite changes in fi ring in different types of neurons might explain the inconsistent multiple unit activity responses that re fl ected mixed unit spikes of pyramidal cells and interneurons.
These data indicate that somatosensory stimulation can enhance the activation of interneurons in the CA1 region. Considering that interneurons have inhibitory effects on primary neurons, the decrease in firing of pyramidal cells induced by somatosensory stimulation could be caused by a decrease in excitability of pyramidal cells or by a decrease in excitatory input from Schaffer collaterals (Miller and Groves, 1977; Herreras et al., 1986). Therefore, we examined changes in the excitability of pyramidal cells under somatosensory stimulation by applying stimulation pulses on the afferent Schaffer collateral pathway with a constant current intensity.
Somatosensory stimulation suppressed the excitability of pyramidal cells in the CA1 region
To investigate the changes in the excitability of pyramidal cells, electrical pulses with a constant current of 0.25-0.35 mA were applied on the Schaffer collaterals in the CA1 region. The test stimulus was applied before and after the onset of tail clamping, as well as after the release of tail clamping. As shown inFigure 4, the stimulus evoked orthodromic population spikes with a large amplitude (7.2 ± 1.8 mV) in the CA1 pyramidal layer before the onset of the tail clamping. In comparison, during tail clamping, the same stimulus evoked population spikes with a signi fi cantly reduced amplitude (5.1 ± 2.5 mV). After the release of tail clamping, the population spike amplitudes returned to the original level (6.8 ± 1.8 mV, n = 12). This result shows that constant afferent inputs induce smaller responses in CA1 pyramidal cells during tail clamping, indicating that somatosensory stimulation can suppress pyramidal cell excitability.
Discussion
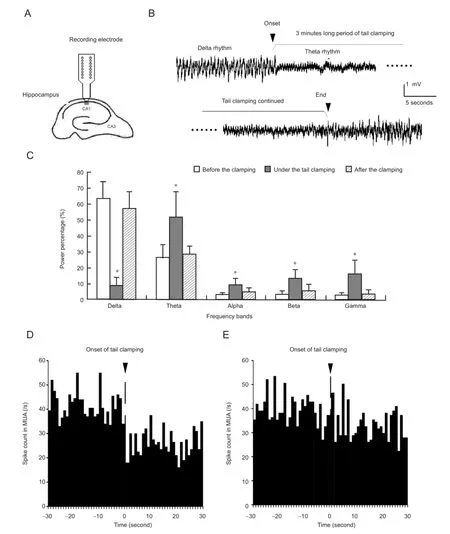
Figure 1 Changes in local fi eld potentials and multiple unit activity (MUA) under somatosensory stimulation.
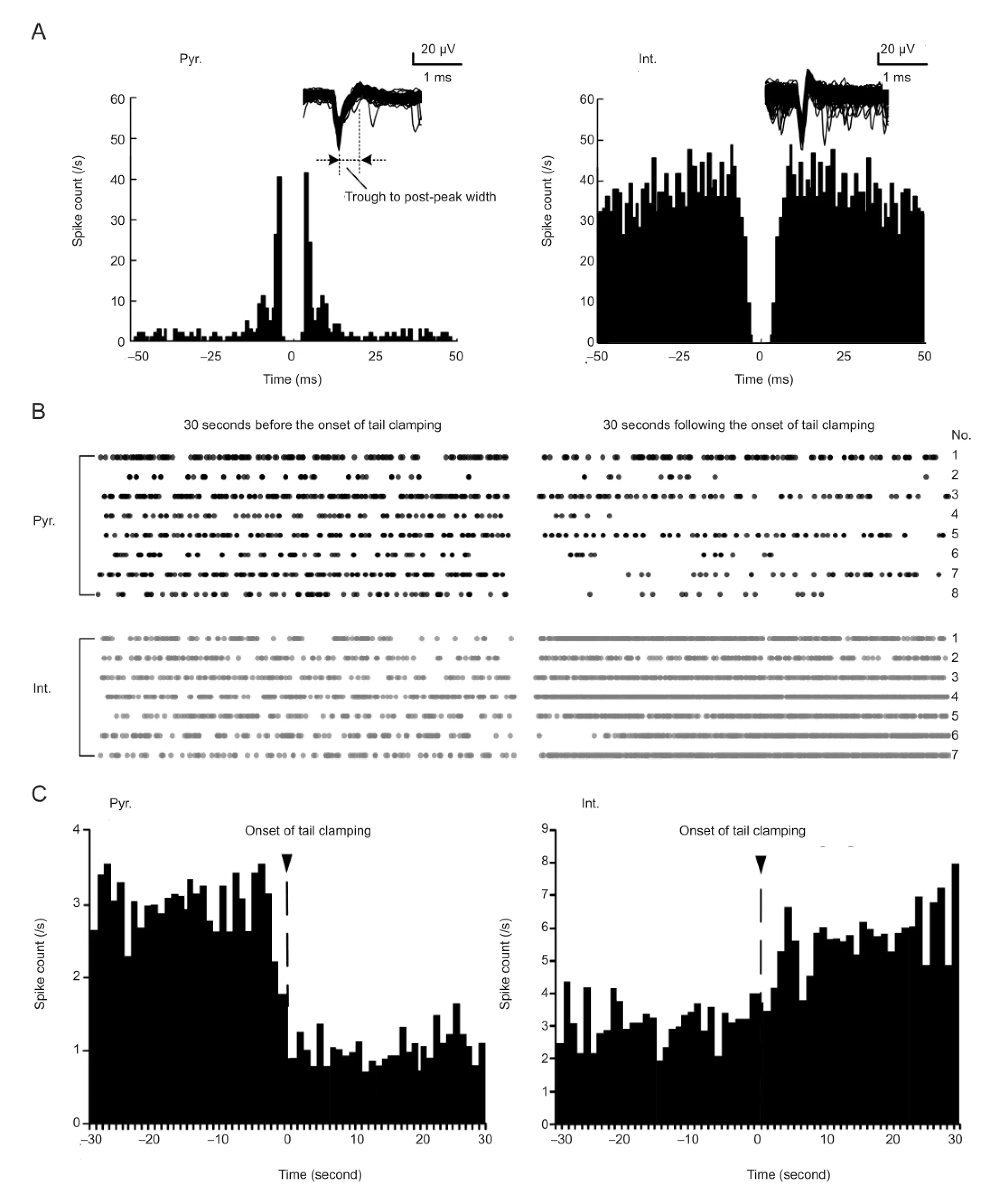
Figure 2 Different responses of pyramidal cells and interneurons induced by somatosensory stimulation.
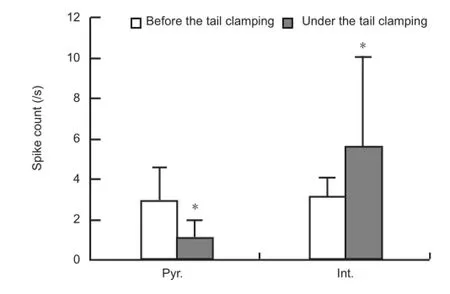
Figure 3 Comparison of the fi ring rates of pyramidal cells (Pyr.) and interneurons (Int.) between the two 30-second periods obtained before and after the onset of tail clamping.
This study shows that somatosensory stimulation changes local fi eld potentials into theta rhythm waveforms and decreases the spike firing of pyramidal cells, while increasing the firing of interneurons. In addition, the attenuation of orthodromic-evoked population spikes indicates a decrease in the excitability of CA1 pyramidal cells by somatosensory stimulation.
Somatosensory input pathways in the brain project to the primary somatosensory cortex, then pass through the entorhinal cortex to enter the hippocampus (Zainos et al., 1997; Melzer et al., 2006). The inputs from the entorhinal cortex to the hippocampal CA1 region can either directly or indirectly (through CA3 and Schaffer collaterals) excite the dendrites of pyramidal cells in the CA1 (Lee et al., 2004; Andersen et al., 2006; Cutsuridis et al., 2010). However, during somatosensory stimulation, the firing of pyramidal cells in the CA1 decreases, instead of increasing, consistent with other studies (Khanna, 1997; Zheng and Khanna, 2001; Bellistri et al., 2013). Local network properties in the CA1 region can explain this phenomenon.
In the CA1, the excitability of pyramidal cells is modulated by local inhibitory circuits consisting of interneurons (Alger and Nicoll, 1982; Papatheodoropoulos and Kostopoulos, 1998; Margineanu and Wülfert, 2000). Somatosensory inputs from both the entorhinal cortex and Schaffer collaterals can excite these interneurons (Lee et al., 2004; Andersen et al., 2006; Cutsuridis et al., 2010). This point is corroborated by the appearance of a strong theta rhythm in the local field potential during somatosensory stimulation, because the firing of interneurons can generate theta rhythms in the CA1 (Alonso and Köhler, 1982; Buzsáki et al., 1986; Toth et al., 1993; Buzsáki, 2002). Presumably, the significantly enhanced activity of interneurons suppresses the excitability of pyramidal cells through local inhibitory circuits, resulting in reduced pyramidal cell activity. In addition, the suppression of orthodromic-evoked population spikes during somatosensory stimulation suggests that pyramidal cell excitability was reduced. Therefore, the present findings suggest that sensory information can decrease rather than increase the activity of pyramidal neurons in the hippocampus.
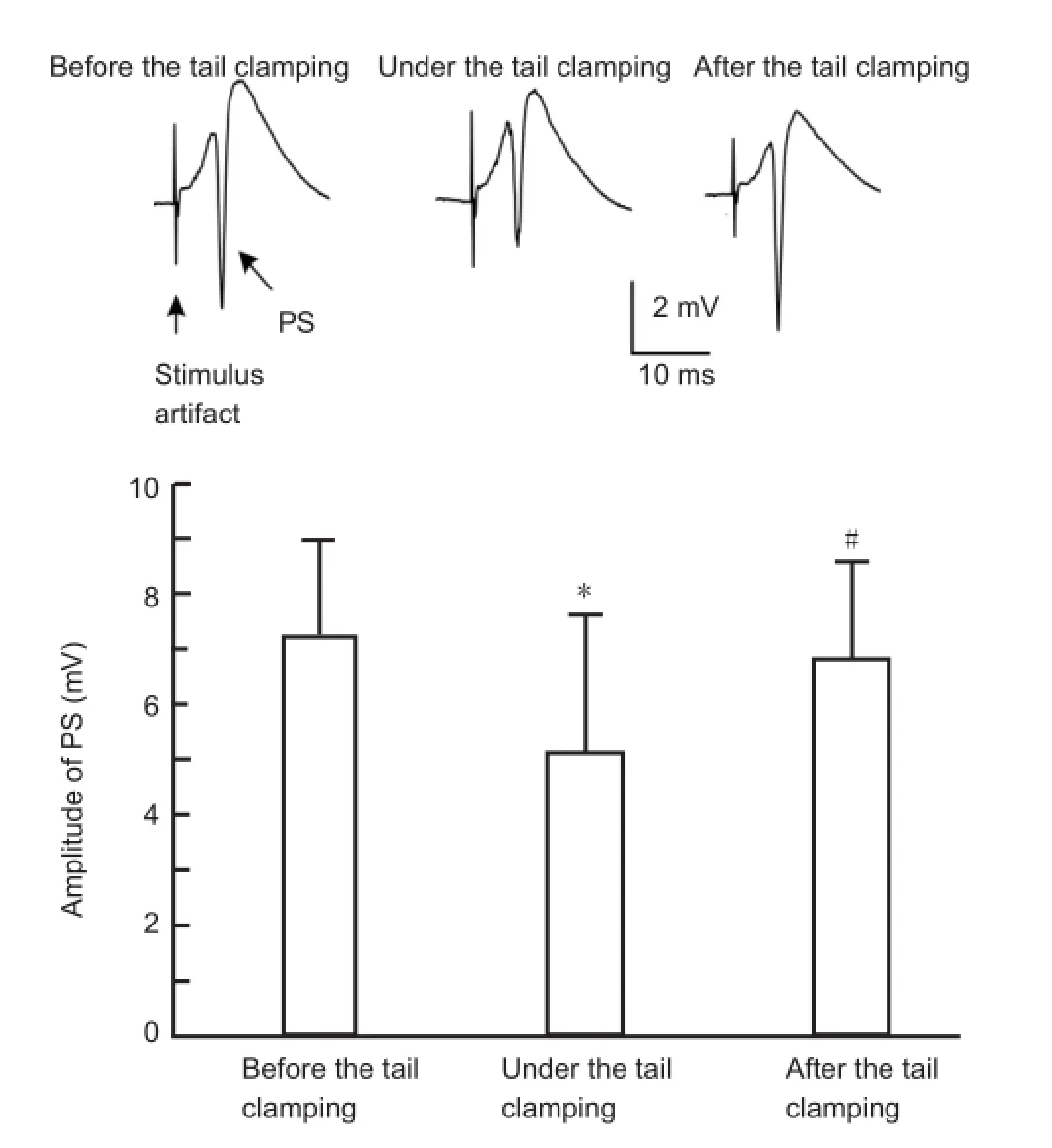
Figure 4 Somatosensory stimulation signi fi cantly decreases the amplitude of orthodromic-evoked population spikes (PSs) in the CA1 region.
The present study suggests that the somatosensory-induced suppression of neuronal excitability under anesthesia may have therapeutic potential for abnormal brain states involving neuronal hyperactivity, such as epilepsy during sleep. Non-rapid eye movement sleep has been shown to facilitate the occurrence of seizures and epileptiform abnormalities (Matos et al., 2010; van Golde et al., 2011). In addition, small-wave desynchronized activity during arousal and rapid eye movement stages can reduce epileptiform activity (Matos et al., 2010). Our experiments were carried out under anesthesia. Large-amplitude slow waves in the delta frequency range (< 2 Hz) dominated the local fi eld potentials during anesthesia, indicating a state that was similar to non-rapid eye movement sleep. The increase in inhibitory interneuron activity during tail clamping characterized by theta-dominated small amplitude local fi eld potentials provides insight into the mechanisms underlying epilepsy suppression. Moreover, a previous study showed that somatosensory stimulation, such as fur stroking, abolishes interictal spike activity in epileptic animals (Lerma et al., 1984). Therefore, somatosensory stimulation may be a potential therapeutic approach for some types of neuronal hyperactivity. However, more investigations with epileptic animals are necessary to evaluate the impact of sensory stimulation on seizure activity.
Although tail clamping may represent a noxious pain stimulus, the alterations in the fi ring of pyramidal cells induced bytail clamping are consistent with previous studies of moderate stimulation such as strokes on paws and whiskers (Miller and Groves, 1977; Vinogradova et al., 1993; Bellistri et al., 2013). Therefore, our results support the notion that different sensory inputs induce similar responses in the hippocampal region because they share common pathways of information processing (Bellistri et al., 2013). By using tail clamping, we can obtain consistent individual neuronal responses, which can facilitate statistical analysis of activity changes in individual neurons in a moderately long period. Therefore, tail clamping appears to be a simple and reliable method for investigating the mechanisms of somatosensory stimulation.
In conclusion, somatosensory stimulation suppresses pyramidal cell excitability and fi ring in the hippocampal CA1 region. Increased inhibition by local interneurons might underlie this effect of the sensory input. Taken together, our findings provide valuable insight into the mechanisms of signal processing in the hippocampus. They also suggest that somatosensory stimulation may have therapeutic potential in brain disorders characterized by neuronal hyperactivity (Lerma et al., 1984).
Author contributions:Wang Y and Feng ZY wrote this manuscript. All authors designed, performed and evaluated the study, and approved the final version of the paper.
Con fl icts of interest:None declared.
Alger BE, Nicoll RA (1982) Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol 328:105-123.
Alonso A, Köhler C (1982) Evidence for separate projections of hippocampal pyramidal and non-pyramidal neurons to different parts of the septum in the rat brain. Neurosci Lett 31:209-214.
Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J (2006) The Hippocampus Book. Oxford: Oxford University Press.
Barthó P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsáki G (2004) Characterization of neocortical principal cells and interneurons by network interactions and extracellular features. J Neurophysiol 92:600-608.
Bellistri E, Aguilar J, Brotons-Mas JR, Foffani G, de la Prida LM (2013) Basic properties of somatosensory-evoked responses in the dorsal hippocampus of the rat. J Physiol 591:2667-2686.
Bermudez Contreras EJ, Schjetnan AGP, Muhammad A, Bartho P, McNaughton BL, Kolb B, Gruber AJ, Luczak A (2013) Formation and reverberation of sequential neural activity patterns evoked by sensory stimulation are enhanced during cortical desynchronization. Neuron 79:555-566.
Bienvenu TC, Busti D, Magill PJ, Ferraguti F, Capogna M (2012) Cell-type-speci fi c recruitment of amygdala interneurons to hippocampal theta rhythm and noxious stimuli in vivo. Neuron 74:1059-1074.
Buzsáki G (2002) Theta oscillations in the hippocampus. Neuron 33: 325-340.
Buzsáki G, Draguhn A (2004) Neuronal oscillations in cortical networks. Science 304:1926-1929.
Buzsáki G, Czopf J, Kondakor I, Kellenyi L (1986) Laminar distribution of hippocampal rhythmic slow activity (RSA) in the behaving rat: current-source density analysis, effects of urethane and atropine. Brain Res 365:125-137.
Csicsvari J, Hirase H, Czurkó A, Mamiya A, Buzsáki G (1999) Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving rat. J Neurosci 19:274-287.
Cutsuridis V, Cobb S, Graham BP (2010) Encoding and retrieval in a model of the hippocampal CA1 microcircuit. Hippocampus 20:423-446.
Deadwyler SA, West MO, Robinson JH. Evoked potentials from the dentate gyrus during auditory stimulus generalization in the rat. Exp Neurol. 1981;71:615-624.
Feng Z, Zheng X, Yu Y, Durand DM (2013) Functional disconnection of axonal fi bers generated by high frequency stimulation in the hippocampal CA1 region in-vivo. Brain Res 1509:32-42.
George P, Charles W (2007) The Rat Brain in Stereotaxic Coordinates. London: Academic Press.
Harris KD, Henze DA, Csicsvari J, Hirase H, Buzsáki G (2000) Accuracy of tetrode spike separation as determined by simultaneous intracellular and extracellular measurements. J Neurophysiol 84:401-414.
Herreras O, Soils J, Lerma J (1986) Abolition of CA1 population spike by sensory stimulation. Exp Brain Res 61:654-657.
Itskov PM, Vinnik E, Diamond ME. Hippocampal representation of touch-guided behavior in rats: persistent and independent traces of stimulus and reward location. PLoS One. 2011;6:e16462.
Khanna S (1997) Dorsal hippocampus field CA1 pyramidal cell responses to a persistent vs an acute nociceptive stimulus and their septal modulation. Neuroscience 77:713-721.
Kloosterman F, Peloquin P, Leung LS (2001) Apical and basal orthodromic population spikes in hippocampal CA1 in vivo show different origins and patterns of propagation. J Neurophysiol 86:2435-2444.
Lee I, Yoganarasimha D, Rao G, Knierim JJ (2004) Comparison of population coherence of place cells in hippocampal sub fi elds CA1 and CA3. Nature 430:456-459.
Lerma J, Herreras O, Munoz D, Solís JM (1984) Interactions between hippocampal penicillin spikes and theta rhythm. Electroencephalogr Clin Neurophysiol 57:532-540.
Lewicki MS (1998) A review of methods for spike sorting: the detection and classi fi cation of neural action potentials. Network 9:R53-78.
Margineanu DG, Wülfert E (2000) Differential paired-pulse effects of gabazine and bicuculline in rat hippocampal CA3 area. Brain Res Bull 51:69-74.
Matos G, Andersen ML, do Valle AC, Tu fi k S (2010) The relationship between sleep and epilepsy: evidence from clinical trials and animal models. J Neurol Sci 295:1-7.
Melzer P, Champney GC, Maguire MJ, Ebner FF (2006) Rate code and temporal code for frequency of whisker stimulation in rat primary and secondary somatic sensory cortex. Exp Brain Res 172:370-386.
Miller SW, Groves PM (1977) Sensory evoked neuronal activity in the hippocampus before and after lesions of the medial septal nuclei. Physiol Behav 18:141-146.
Papatheodoropoulos C, Kostopoulos G (1998) Development of a transient increase in recurrent inhibition and paired-pulse facilitation in hippocampal CA1 region. Brain Res Dev Brain Res 108:273-285.
Pereira A, Ribeiro S, Wiest M, Moore LC, Pantoja J, Lin SC, Nicolelis MA (2007) Processing of tactile information by the hippocampus. Proc Natl Acad Sci U S A 104:18286-18291.
Ranck JB (1973) Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats: Part I. Behavioral correlates and fi ring repertoires. Exp Neurol 41:462-531.
Sceniak MP, Maciver MB. Cellular actions of urethane on rat visual cortical neurons in vitro. J Neurophysiol. 2006;95: 3865-3874.
Toth K, Borhegyi Z, Freund TF (1993) Postsynaptic targets of GABAergic hippocampal neurons in the medial septum-diagonal band of broca complex. J Neurosci 13:3712-3724.
van Golde EG, Gutter T, de Weerd AW (2011) Sleep disturbances in people with epilepsy; prevalence, impact and treatment. Sleep Med Rev 15:357-368.
Vinogradova OS, Brazhnik ES, Kitchigina VF, Stafekhina VS (1993) Acetylcholine, theta-rhythm and activity of hippocampal neurons in the rabbit-IV. Sensory stimulation. Neuroscience 53:993-1007.
Welch PD (1967) The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modi fi ed periodograms. IEEE Trans Acoust 15:70-73.
Zainos A, Merchant H, Hernández A, Salinas E, Romo R (1997) Role of primary somatic sensory cortex in the categorization of tactile stimuli: effects of lesions. Exp Brain Res 115:357-360.
Zheng F, Khanna S (2001) Selective destruction of medial septal cholinergic neurons attenuates pyramidal cell suppression, but not excitation in dorsal hippocampus fi eld CA1 induced by subcutaneous injection of formalin. Neuroscience 103:985-998.
Copyedited by Patel B, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.135316
http://www.nrronline.org/
Accepted: 2014-05-03
The hippocampal region of the brain is important for encoding environment inputs and memory formation. However, the underlying mechanisms are unclear. To investigate the behavior of individual neurons in response to somatosensory inputs in the hippocampal CA1 region, we recorded and analyzed changes in local fi eld potentials and the fi ring rates of individual pyramidal cells and interneurons during tail clamping in urethane-anesthetized rats. We also explored the mechanisms underlying the neuronal responses. Somatosensory stimulation, in the form of tail clamping, changed local fi eld potentials into theta rhythm-dominated waveforms, decreased the spike fi ring of pyramidal cells, and increased interneuron fi ring. In addition, somatosensory stimulation attenuated orthodromic-evoked population spikes. These results suggest that somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region. Increased inhibition by local interneurons might underlie this effect. These fi ndings provide insight into the mechanisms of signal processing in the hippocampus and suggest that sensory stimulation might have therapeutic potential for brain disorders associated with neuronal hyperexcitability.
- 中国神经再生研究(英文版)的其它文章
- Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment
- High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction
- A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis
- Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons
- Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemiareperfusion injury in aged rats
- Neuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor

