Neuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor
Yong Liu, Suiqiang Zhu Yunfu Wang, Jingquan Hu, Lili Xu, Li Ding, Guangjian Liu
1 Department of Neurology, Tongji Hospital Af fi liated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
2 Department of Neurology, Taihe Hospital Af fi liated to Hubei University of Medicine, Shiyan, Hubei Province, Chi-na
Neuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor
Yong Liu1,2, Suiqiang Zhu1, Yunfu Wang2, Jingquan Hu2, Lili Xu2, Li Ding2, Guangjian Liu2
1 Department of Neurology, Tongji Hospital Af fi liated to Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei Province, China
2 Department of Neurology, Taihe Hospital Af fi liated to Hubei University of Medicine, Shiyan, Hubei Province, Chi-na
Neuroprotection by ischemic preconditioning has been confirmed by many studies, but the precise mechanism remains unclear. In the present study, we performed cerebral ischemic preconditioning in rats by simulating a transient ischemic attack twice (each a 20-minute occlusion of the middle cerebral artery) before inducing focal cerebral infarction (2 hour occlusion-reperfusion in the same artery). We also explored the mechanism underlying the neuroprotective effect of ischemic preconditioning. Seven days after occlusion-reperfusion, tetrazolium chloride staining and immunohistochemistry revealed that the infarct volume was signi fi cantly smaller in the group that underwent preconditioning than in the model group. Furthermore, vascular endothelial growth factor immunoreactivity was considerably greater in the hippocampal CA3 region of preconditioned rats than model rats. Our results suggest that the protective effects of ischemic preconditioning on focal cerebral infarction are associated with upregulation of vascular endothelial growth factor.
nerve regeneration; brain injury; transient ischemic attack; ischemic preconditioning; ischemia-reperfusion; focal cerebral infarction; infarct volume ratio; vascular endothelial growth factor; protection; mechanism; neural regeneration
Liu Y, Zhu SQ, Wang YF, Hu JQ, Xu LL, Ding L, Liu GJ. NNeuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor. Neural Regen Res. 2014;9(11):1117-1121.
Introduction
Kitagawa et al. (1990) found that the brain has ischemic tolerance, and proposed the concept of ischemic preconditioning. Numerous studies have demonstrated the neuroprotective effects of cerebral ischemic preconditioning, but, to our knowledge, none have determined whether ischemic preconditioning can reduce infarct size. There are many reports detailing methods to perform ischemic preconditioning, including hypoxia, intense heat, low temperature and hyperbaric oxygen, but some of these methods go beyond the scope of “ischemia” (Kato et al., 1994; Benjamin and Mc-Millan, 1998; Gamperl and Farrell, 2004; Nilsson and Renshaw, 2004; Shankaran et al., 2005; Bickler and Buck, 2007; Horowitz, 2007).
Studies exploring the mechanism of ischemic preconditioning are often based on speculation, and are even rarer in China than in the western world. Some researchers believe that ischemic preconditioning promotes the expression of many growth factors, such as epidermal growth factor, brain-derived neurotrophic factor, vascular endothelial growth factor and glial-derived growth factor (Dempsey et al., 2003), and that these factors are involved in the induction of ischemic tolerance and improve the symptoms of ischemic stroke (Liu et al., 2005).
Here, we used an ischemic preconditioning method that mimics a transient ischemic attack to observe the effects of preconditioning on cerebral infarction. We also measured expression of vascular endothelial growth factor to examine the mechanism underlying the protective effect of ischemic preconditioning on brain tissue.
Materials and Methods
Experimental animals and experimental groups
Forty- fi ve adult, female, speci fi c pathogen free Sprague-Dawley rats, weighing 160-220 g, were purchased from the Experimental Animal Center of Hubei Medical College in China (SCXK(E)2011-0008). All rats were habituated to their environment and fed normally for 1 week. In the night before the experiment, the animals were fasted, but had free access to water. They were then randomly allocated to a preconditioning group (n = 18), a model group (n = 18) and a sham-operated group (n = 9).
Pretreatment group: 20-minute ischemic preconditioning was carried out on days 1 and 4, before models of middle cerebral artery occlusion and reperfusion (abbreviated as cerebral ischemia-reperfusion) were established on day 8.
Sham-operated group: rats underwent an identical procedure to the pretreatment group, except that once the ca-rotid artery was exposed, and the incision was closed again without occlusion of the artery. The rats were then returned to their home cages before being sacri fi ced at the end of the experiment.
Model group: rats underwent the same procedures as the sham-operated group; the neck incision was made to expose the carotid artery then closed at 1 week. Similarly to the pretreatment group, the cerebral ischemia- reperfusion model was established on day 8.
Ischemic preconditioning
Reversible middle cerebral artery occlusion was performed on days 1 and 4, using a modi fi ed suture method. On day 1, rats were weighed and anesthetized with an intraperitoneal injection of 1% sodium pentobarbital (30 mg/kg; Hongyunlong Biological Technology Co., Ltd., Wuhan, China). Fur on the neck was shaved and routinely disinfected. A longitudinal incision of about 1.2 cm was made on the righthand side of the neck. The right common carotid, external carotid and internal carotid arteries were isolated. A seton was placed at the proximal end of the external carotid artery. The artery was ligated at the distal end, and a small hole was cut with ophthalmic scissors between the seton and the ligation. A paraffin-treated nylon fishing wire (0.3 mm in diameter) was inserted into the carotid artery 20 mm from the bifurcation of the external and internal carotid, and then pulled outwards about 2 mm to reach resistance. The anterior cerebral artery was not blocked. The wire remained in place for 20 minutes before being removed from the external carotid artery, which was then ligated and fi xed. The incision was sutured and the rats were allowed to recover. On day 4, a similar procedure was conducted. The external carotid artery was isolated and the ligation thread at the proximal end of the external carotid artery was loosened. A nylon fishing line was inserted into the same position as on day 1, held in place for 20 minutes, pulled back to the external carotid artery, tied, and fi xed. The incision was sutured and the rats were allowed to recover. Rats were excluded from the experiment if nerve defects were caused by the pretreatment. Sham-operated animals underwent the same procedure as the preconditioned animals, except that a neck incision was made to expose the carotid artery, and then closed, without occlusion of the artery.
Establishment of a rat model of cerebral ischemia/ reperfusion
Surgery for cerebral ischemia/reperfusion was similar to that for ischemic preconditioning. The nylon fishing wire was inserted into the same position but kept in place for 2 hours, and then pulled out. The proximal end of the external carotid artery was ligated. The occurrence of hemiplegia indicated that the model was successfully established.
Calculation of infarct volume percentage
The rats were decapitated at 7 days after middle cerebral artery occlusion. Brains were frozen at -20°C for 3 minutes, and cut into fi ve coronal slices of approximately 2 mm thickness. The slices were stained with 2,3,5-triphenyltetrazolium chloride (TTC) (Shanghai Third Reagent Factory, Shanghai, China). The infarct volume was calculated as a percentage using a medical image processing system (TJTY-300, Tongji Sun Company, Wuhan, China) in accordance with a previous study (Liu et al., 2003). The total infarct volume in each slice was divided by the total brain tissue volume of each slice, to obtain the volume of infarct as a percentage of total tissue.
Volume percentage = (s1× h + s2× h + s3× h + s4× h + s5× h)/(S1× h + S2× h + S3× h + S4× h + S5× h) × 100% = (s1+ s2+ s3+ s4+ s5) × h/(S1+ S2+ S3+ S4+ S5) × h × 100% = (s1+ s2+ s3+ s4+ s5)/(S1+ S2+ S3+ S4+ S5) × 100%, where“s” represents infarct area, S represents the area of the whole brain slice, h represents the thickness of the brain slices, and the numbers 1-5 identify each slice.
Vascular endothelial growth factor immunohistochemistry
The third brain slice was fixed in 4% paraformaldehyde phosphate buffer for 24 hours at room temperature and dehydrated with graded ethanol. The slice was then treated with dimethylbenzene, embedded in paraf fi n, and cut into sections of 5 µm thickness. These sections were baked then rehydrated and antigen retrieval was performed before incubation with 1:100 vascular endothelial growth factor mouse monoclonal antibody for 60 minutes at 37°C and 12 hours at 4°C (Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China), followed by a streptavidin peroxidase kit (goat anti-mouse IgG; Beijing Zhongshan Biotechnology Co., Ltd.) and 3,3′-diaminobenzidine chromogenic staining kit (Beijing Zhongshan Biotechnology Co., Ltd.), carried out according to the manufacturers’instructions. Finally, the sections were dehydrated and sealed. The morphology of stained cells was observed under a light microscope (Olympus, Tokyo, Japan). The number of positive cells (cytoplasm stained brownish yellow) around the infarct site in 10 high-powered fi elds of view (× 400) was calculated for each specimen. The mean value was taken as the density of positive cells in the specimen. Positive cells were also calculated in the corresponding regions in the sham-operated group using a medical image processing system (TJTY-300; Tongji Sun Company).
Statistical analysis
All data were expressed as mean ± SD. The mean value of each group was compared and analyzed with one-way analysis of variance and two sample t-test, using SPSS 15.0 software (SPSS, Chicago, IL, USA). α = 0.05 was set as the standard.
Results
Ischemic preconditioning reduces infarct volume in rats
TTC staining revealed that at 7 days after model establishment, infarct volume percentage was smaller in rats that had undergone preconditioning than in those that had not. In the sham-operated group, the entire brain slice was stained red, indicating viable tissue, and no infarcts were visible.In the model and pretreatment groups, white (unstained) infarcts of different sizes were observed in the blood supply area of the right middle cerebral artery (Figure 1). The infarct size was expressed as the ratio of infarct volume to the volume of whole brain (percentage of cerebral infarct). Infarcts were smaller in the pretreatment group (12.17 ± 2.64%) than in the model group (23.41 ± 3.91%; P < 0.01).
Effects of ischemic preconditioning on vascular endothelial growth factor immunoreactivity in rat brain after cerebral infarction
Vascular endothelial growth factor-immunoreactive cells in the hippocampal CA3 region were mainly observed in the cytoplasm and membrane of neurons, and stained brownish yellow. Stained cells were of similar size in each group. A few scattered positive cells with normal morphology were found in both hemispheres of rats from the sham-operated group, and in the contralateral hemisphere of rats from the model and pretreatment groups. However, many vascular endothelial growth factor-immunoreactive cells with irregular morphology were observed around the infarct zones (cortex, medulla and hippocampus) in the ipsilateral hemisphere. In the model group, the pathological alterations were apparent. In the pretreatment group, the number of vascular endothelial growth factor-immunoreactive cells in the hippocampal CA3 region was greater (37.65 ± 3.96 per high-powered field) than in the model group (26.42 ± 4.05 per high-powered fi eld) and sham-operated group (4.39 ± 1.09 per high-powered fi eld; P < 0.01;Figure 2).
Discussion
Ischemic preconditioning was first found by Murry in the dog model of myocardial ischemia (Murry, et al., 1986). Four years later, Kitagawa proposed the concept of ischemic preconditioning in studies on cerebral ischemia in gerbils, and believed that after one or more transient non-lethal ischemic episodes, brain tissue could effectively mobilize endogenous protective mechanisms to reduce damage to brain cells in the event of severe cerebral ischemia. This highlighted the phenomenon of ischemic tolerance in the brain. There have been many studies on ischemic preconditioning, especially outside China. The methods of obtaining ischemic preconditioning are abundant and varied, including chemical pretreatment, drugs such as iso fl urane, hypoxia, intense heat, low temperature and high pressure oxygen (Xiong et al., 2000; Mastronardi and Ca fi ero, 2001; Dong et al., 2002), but they are not speci fi c enough, and some of the methods go beyond the scope of ischemia. In the present study, we performed true ischemic preconditioning by simulating a clinical transient ischemic attack. The method we employed was based on the middle cerebral artery occlusion-reperfusion model, but the occlusion time was considerably shorter, at 20 minutes. In the procedure, the nylon wire was inserted into the deepest end (1.8-2.0 mm) and then pulled back 1.0-2.0 mm, so as not to occlude the anterior cerebral artery or cause large ischemia. After withdrawal of the suture, left limb paresis was observed, and the rats recovered fully within 2 hours, which suggested successful ischemic preconditioning by transient ischemia. The smaller infarct volume and elevated expression of vascular endothelial growth factor observed in the pretreatment group supports the feasibility of this protocol. When performing such a procedure, gentle movements should be ensured to minimize surgical trauma, and the protection of blood vessels is especially important.
In the present experiment, TTC staining clearly revealed a white infarct area. The percentage infarct volume of rats in the pretreatment group was significantly smaller than that in the model group, providing further evidence, consistent with the literature, of the protective effect on brain tissue of ischemic preconditioning by transient ischemic attack.
Several studies have explored the mechanism underlying the protective effects of ischemic preconditioning. The technique promotes the expression of many growth factors, such as insulin-like growth factor 1, fi broblast growth factor 2, transforming growth factor β1, epidermal growth factor, brain-derived neurotrophic factor, erythropoietin, vascular endothelial growth factor, glial-derived growth factor and platelet-derived growth factor (Dempsey et al., 2003). These growth factors are involved in the generation of ischemic tolerance and improve the symptoms of an ischemic stroke model (Liu et al., 2005). They may offer protection through resistance to apoptosis and in fl ammation, but also mediate nerve repair. Gustavsson et al. (2007) believed that, during cerebral ischemia, the generation of new blood vessels was stimulated through the regulation of vascular endothelial growth factor and erythropoietin cytokines by hypoxia-induced factor-1, and ischemic preconditioning reduced the decline of cerebral blood fl ow in severe cerebral ischemia.
Vascular endothelial growth factor is a highly specified mitogen of vascular endothelial cells. Upon binding to its receptor, it speeds up the formation of new blood vessels by promoting the proliferation and migration of endothelial cells; it also enhances vascular permeability (Claesson and Welsh, 2013; Dejana and Vestweber, 2013; Goel and Mercurio, 2013; Kim et al., 2013; Miller et al., 2013; Parsons and Foley, 2013; Terrasi et al., 2013; Jeong et al., 2014; Kim et al., 2014; Liegl et al., 2014; Küppers et al., 2014; Zimmerman et al., 2014). However, the mechanism by which vascular endothelial growth factor acts is still unclear. It probably alters the extracellular environment and contributes to angiogenesis by inducing endothelial cells to express plasminogen activator, plasminogen activator inhibitor-1, tissue factor, matrix collagenase, and factor VIII, ultimately increasing the permeability of blood vessels (Goldberg and Schneider, 1999). The interaction of vascular endothelial growth factor and Flk-1 increases the production of nitric oxide, and, by activating cyclooxygenase, nitric oxide then stimulates the generation of prostacyclin, which enhances blood vessel permeability (Murohara et al., 1998). Noiri and colleagues (1998) showed that vascular endothelial growth factor induces the release of nitric oxide from microvascular endothelial cells, which, in turn, stimulates the proliferation and migration of endothelial cells. Hypoxia or other damage can increase the expression of vascular endothelial growth factor inendothelial cells and/or vascular smooth muscle cells, and elevate vascular endothelial growth factor activation in endothelial cells through autocrine or paracrine signaling to increase the generation of nitric oxide in endothelial cells. The increased nitric oxide reacts to hypoxic smooth muscle cells to lower vascular endothelial growth factor inside. Negative feedback regulation like this ensures the rapid restoration of damaged or hypoxic blood vessel wall while preventing the generation of harmful effects caused by vascular endothelial growth factor over-expression (Bermudez et al., 2011; Dong et al., 2011; Duan et al., 2012; Jurasz et al., 2012; Yang et al., 2012).
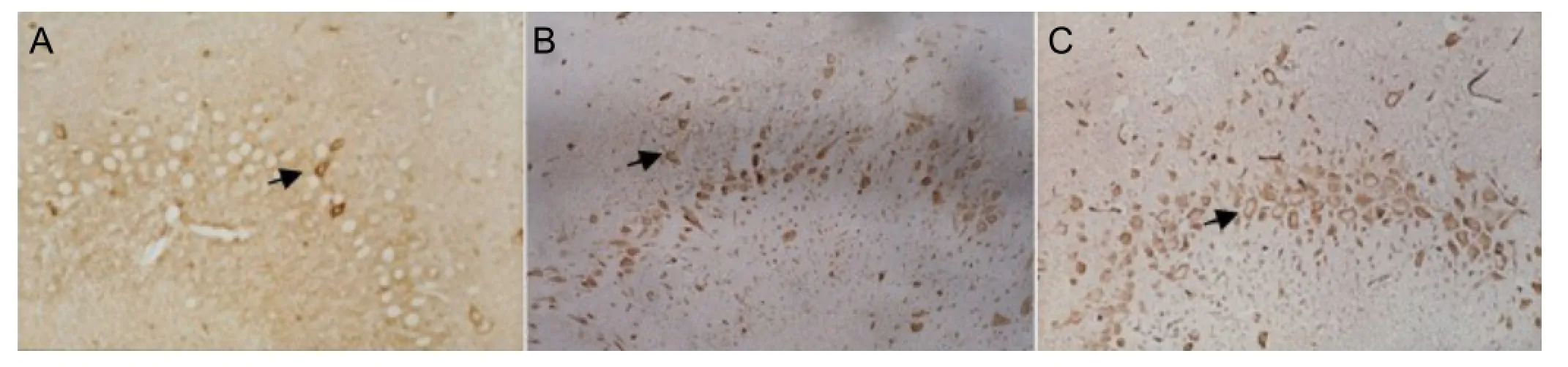
Figure 2 Effects of ischemic preconditioning on vascular endothelial growth factor protein expression in the hippocampal CA3 region of cerebral infarction rats (immunohistochemical staining, light microscope, × 400).
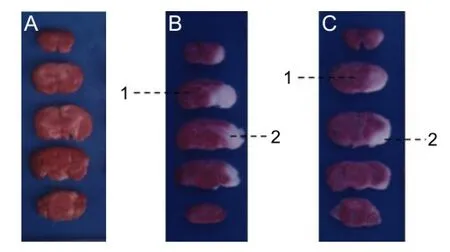
Figure 1 2,3,5-Triphenyltetrazolium chloride (TTC) staining showing effects of ischemic preconditioning on infarct volume in rats.
Results from the present study show that vascular endothelial growth factor expression is greater in the neurons adjacent to the infarct site in rats, indicating that hypoxic and ischemic injuries stimulate the expression of vascular endothelial growth factor. When the cerebral infarction occurred after two sessions of ischemic preconditioning, vascular endothelial growth factor expression in neurons surrounding the infarct site was greater, the infarct was smaller, and there was considerably less neuronal damage. These results suggest that vascular endothelial growth factor played a role in the protection of brain tissue and nerve cells from ischemic preconditioning.
In ischemic preconditioning, vascular endothelial growth factor might achieve the protection of brain tissues in the following ways: (1) Stimulation of blood vessel proliferation, which ensures the blood supply of the penumbra when cerebral infarction occurs. (2) Protection of ischemia-damaged brain tissues by protecting endothelial cells and blood vessels. Alon et al. (1995) suggested that vascular endothelial growth factor avoided programmed death of endothelial cells and prevented the disappearance of capillaries. Ku et al. (1993) showed that vascular endothelial growth factor protected ischemic brain tissue through the generation of nitric oxide, which would in turn relax vascular smooth muscle and promote the recovery of blood fl ow. If endothelial cells and blood vessels are normal or nearly normal, there would be a reduced generation of intercellular adhesion molecules, such as intercellular adhesion molecule-1. The adhesion and infiltration of peripheral monocytes will be impaired, the in fl ammation of ischemic brain tissue will be alleviated, and ischemic brain damage will be reduced. (3) Anti-apoptosis (Zhang et al., 2007). Apoptosis is a major form of cell death within the penumbra. Ischemic preconditioning can reduce the infarct volume, indicating that vascular endothelial growth factor may inhibit neuronal apoptosis directly or indirectly; for example, by inhibiting the expression of a pro-apoptotic gene or promoting the expression of an anti-apoptosis gene.
Through the simulation of the clinical features of transient ischemic attack, we induced ischemic preconditioning in the present study, to allow the objective evaluation of its effects on brain tissue. A preliminary study of the mechanism underlying ischemic preconditioning in the protection of brain tissue was also conducted in this study. However, ischemic preconditioning measures of transient ischemic attack should be further improved to be simple and easy to operate. The mechanism of ischemic preconditioning protecting brain tissue is complex, and deserves further investigation.
Author contributions:Liu Y and Zhu SQ designed the study. Liu Y, Wang YF, Hu JQ, Xu LL and Ding L performed the experiment. Liu GJ analyzed data. Liu Y wrote the manuscript. All authors approved the final version of the paper.
Con fl icts of interest:None declared.
Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E (1995) Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med 1:1024-1028.
Benjamin IJ, McMillan DR (1998) Stress (heat shock) proteins molecular chaperones in cardiovascular biology and disease. Circ Res 83:117-132.
Bermudez O, Jouandin P, Rottier J, Bourcier C, Gimond C (2011) Post transcriptional regulation of the DUSP6/MKP 3 phosphatase by MEK/ERK signaling and hypoxia. J Cell Physiol 226:276-284.
Bickler PE, Buck LT (2007) Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol 69:145-170.
Claesson L, Welsh M (2013) VEGFA and tumour angiogenesis. J Intern Med 273:114-127.
Dejana E, Vestweber D (2013) The role of VE-cadherin in vascular morphogenesis and permeability control. Prog Mol Biol Transl Sci 116:119-144.
Dempsey RJ, Sailor KA, Bowen KK, Türeyen K, Vemuganti R (2003) Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem 87:586-597.
Dong H, Xiong L, Zhu Z, Chen S, Hou L, Sakabe T (2002) Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology 96:907-912.
Dong X, Wang YS, Dou GR, Hou HY, Shi YY, Zhang R, Ma K, Wu L, Yao LB, Cai Y (2011) In fl uence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS One 6:e18481.
Duan QL, Wang XX, Gong W, Ni L, Chen C, He XX, Chen FQ, Yang L, Wang PH, Wang DW (2012) ER stress negatively modulates the expression of the miR-199a/214 cluster to regulates tumor survival and progression in human hepatocellular cancer. PLoS One 7:e31518.
Gamperl AK, Farrell A (2004) Cardiac plasticity in fi shes: environmental in fl uences and intraspeci fi c differences. J Exp Biol 207:2539-2550. Goel HL, Mercurio AM (2013) VEGF targets the tumour cell. Nat Rev Cancer 13:871-882.
Goldberg M, Schneider T (1999) Similarities between the oxyensening mechanisms regulating the expression of VEGF and erythropoietin. J Biol Chem 269:435.
Gustavsson M, Mallard C, Vannucci SJ, Wilson MA, Johnston MV, Hagberg H (2007) Vascular response to hypoxic preconditioning in the immature brain. J Cereb Blood Flow Metab 27:928-938.
Horowitz M (2007) Heat acclimation and cross-tolerance against novel stressors: genomic-physiological linkage. Prog Brain Res 162:373-392.
Jeong W, Kim J, Bazer FW, Song G (2014) Stimulatory effect of vascular endothelial growth factor on proliferation and migration of porcine trophectoderm cells and their regulation by the phosphatidylinositol-3-kinase-AKT and mitogen-activated protein kinase cell signaling pathways. Biol Reprod 90:50.
Jurasz P, Yurkova N, Kirshenbaum L, Stewart DJ (2012) VEGF masks BNIP3-mediated apoptosis of hypoxic endothelial cells. Angiogenesis 14:199-207.
Kato H, Liu Y, Kogure K, Kato K (1994) Induction of 27-kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res 634:235-244.
Kim KM, Kim NS, Kim J, Park JS, Yi JM, Lee J, Bang O (2013) Magnolol suppresses vascular endothelial growth factor-induced angiogenesis by inhibiting Ras-dependent mitogen-activated protein kinase and phosphatidylinositol 3-kinase/Akt signaling pathways. Nutr Cancer 65:1245-1253.
Kim S, Lee Y, Kim N, Hwang Y, Hwang B, Min J, Koh S (2014) Pancreatic adenocarcinoma upregulated factor, a novel endothelial activator, promotes angiogenesis and vascular permeability. Oncogene 32:3638-3647.
Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K (1990) Ischemic tolerance’phenomenon found in the brain. Brain Res 528:21-24.
Ku DD, Zaleski JK, Liu S, Brock TA (1993) Vascular endothelial growth factor induces EDRF-dependent relaxation in coronary arteries. Am J Physiol-heart Circ Physiol 265:H586-592.
Küppers V, Vockel M, Nottebaum AF, Vestweber D (2014) Phosphatases and kinases as regulators of the endothelial barrier function. Cell Tissue Res 355:577-586.
Liegl R, Koenig S, Siedlecki J, Haritoglou C, Kampik A, Kernt M (2014) Temsirolimus inhibits proliferation and migration in retinal pigment epithelial and endothelial cells via mTOR inhibition and decreases VEGF and PDGF expression. PLoS One 9:e88203.
Liu J, Narasimhan P, Yu F, Chan PH (2005) Neuroprotection by hypoxic preconditioning involves oxidative stress-mediated expression of hypoxia-inducible factor and erythropoietin. Stroke 36:1264-1269.
Mastronardi P, Cafiero T (2001) Rational use of opioids. Minerva Anestesiol 67:332-337.
Miller JW, Le Couter J, Strauss EC, Ferrara N (2013) Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology 120:106-114.
Murohara T, Horowitz JR, Silver M, Tsurumi Y, Chen D, Sullivan A, Isner JM (1998) Vascular endothelial growth factor/vascular permeability factor enhances vascular permeability via nitric oxide and prostacyclin. Circulation 97:99-107.
Murry CE, Jennings RB, Reimer KA (1986) Preconditioning with ischemia:a delay of lethal cell injury in ischemic myocardium. Circulation 74:1124-1136
Nilsson GE, Renshaw GM (2004) Hypoxic survival strategies in two fi shes: extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J Exp Biol 207:3131-3139.
Noiri E, Lee E, Testa J, Quigley J, Col fl esh D, Keese CR, Giaever I, Goligorsky MS (1998) Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol-cell 274:C236-244.
Parsons B, Foley E (2013) The drosophila platelet-derived growth factor and vascular endothelial growth factor-receptor related (Pvr) protein ligands Pvf2 and Pvf3 control hemocyte viability and invasive migration. J Biol Chem 288:20173-20183.
Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD (2005) Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. New Engl J Med 353:1574-1584.
Terrasi M, Bazan V, Caruso S, Insalaco L, Amodeo V, Fanale D, Corsini LR, Contaldo C, Mercanti A, Fiorio E (2013) Effects of PPARγ agonists on the expression of leptin and vascular endothelial growth factor in breast cancer cells. J Cell Physiol 228:1368-1374.
Xiong L, Zhu Z, Dong H, Hu W, Hou L, Chen S (2000) Hyperbaric oxygen preconditioning induces neuroprotection against ischemia in transient not permanent middle cerebral artery occlusion rat model. Chin Med J (Engl) 113:836-839.
Yang J, Caldwell RB, Behzadian MA (2012) Blockade of VEGF-induced GSK/β-catenin signaling, uPAR expression and increased permeability by dominant negative p38α. Exp Eye Res 100:101-108.
Zhang Y, Park TS, Gidday JM (2007) Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol-heart Circ Physiol 292:H2573-2581.
Zimmerman MW, McQueeney KE, Isenberg JS, Pitt BR, Wasserloos KA, Homanics GE, Lazo JS (2014) PTP4A3 phosphatase promotes VEGF signaling and enables endothelial cell motility. J Biol Chem 289:5904-5913.
Copyedited by Slone-Murphy J, Norman C, Wang J, Qiu Y, Li CH, Song LP, Zhao M
10.4103/1673-5374.135313
Yong Liu, Ph.D., Department of
Neurology, Taihe Hospital Affiliated to Hubei University of Medicine, Shiyan 442000, Hubei Province, China,
taihly@sina.com
http://www.nrronline.org/
Accepted: 2014-04-01
- 中国神经再生研究(英文版)的其它文章
- Acupuncture at the Taixi (KI3) acupoint activates cerebral neurons in elderly patients with mild cognitive impairment
- High matrix metalloproteinase-9 expression induces angiogenesis and basement membrane degradation in stroke-prone spontaneously hypertensive rats after cerebral infarction
- A viral vector expressing hypoxia-inducible factor 1 alpha inhibits hippocampal neuronal apoptosis
- Somatosensory stimulation suppresses the excitability of pyramidal cells in the hippocampal CA1 region in rats
- Heavy ion and X-ray irradiation alter the cytoskeleton and cytomechanics of cortical neurons
- Potential targets for protecting against hippocampal cell apoptosis after transient cerebral ischemiareperfusion injury in aged rats

