Solubility of Ammonia in Ethylene Glycol Between 303 K and 323 K under Low Pressure from 0.030 to 0.101 MPa*
(周桓)**(张帅)(高飞)(白晓琴)(沙作良)
Tianjin Key Laboratory of Marine Resources and Chemistry, Tianjin University of Science and Technology, Tianjin 300457, China
Solubility of Ammonia in Ethylene Glycol Between 303 K and 323 K under Low Pressure from 0.030 to 0.101 MPa*
ZHOU Huan(周桓)**, ZHANG Shuai(张帅), GAO Fei(高飞), BAI Xiaoqin(白晓琴)and SHA Zuoliang(沙作良)
Tianjin Key Laboratory of Marine Resources and Chemistry, Tianjin University of Science and Technology, Tianjin 300457, China
The solubility of ammonia in ethylene glycol is measured by an isothermal solubility equilibrium method at temperatures of (303.2, 308.2, 313.2, 318.2 and 323.2) K and total pressures of (0.030, 0.040, 0.050, 0.060, 0.070, 0.080, 0.090 and 0.101) MPa. The molality of ammonia in ethylene glycol ranges from 1.925 mol·kg−1to 8.265 mol·kg−1. The experimental results are used to determine Henry’s law constant of ammonia in ethylene glycol. Furthermore, experimental data are correlated by applying the thermodynamic model on the basis of extended Raoult’s law, extended Henry’s law, corresponding-states correlations and Pitzer’s molality scale based equation. The overall average relative deviation between the calculated data and the experimental data of Henry’s law constant and ammonia solubility are 2.029% and 2.164% respectively.
gas solubility, ammonia, ethylene glycol, negative pressure
1 INTRODUCTION
The solubility of gases in aqueous as well as non-aqueous mixed solvents must be known for the design of many separation or reaction processes with gas as a reactant or product [1, 2]. For example, one of the approaches to producing magnesium chloride hexammoniate (MgCl2·6NH3), the intermediate of high-purity anhydrous magnesium chloride (MgCl2) and a solid high-density ammonia storage carrier, is that magnesium chloride reacts with ammonia gas in organic solvent, such as methanol, ethanol, glycol or their mixed solvents by reaction crystallization [3-6]. The concentration of ammonia, as a reactant, in a reaction crystallization process must be kept in an appropriate range, otherwise, the reaction will result in byproduct biglycollate biammoniate magnesium chloride (MgCl2·2C2H6O2·2NH3) or other complexes [3, 5]. The solubility of ammonia in the single or mixed solvent, or MgCl2contained solution and the effect of pressure and temperature are significant to the reaction mechanism and kinetics, operation of crystallization process or the recovery of mother liquid. The work presented here is restricted to one of the interesting subsystems: the solubility of ammonia in pure ethylene glycol.
It is not easy to predict the solubility of ammonia in aqueous, organic solvent, salt-free or salt-containing solutions by solution theory such as regular solution theory and by cubic equation of state, due to the hydrogen-bonded or highly polar fluids, or chemical reaction equilibrium. The experimental investigation and thermodynamic model for ammonia solubility are active in recent years. For example, Rumpf and Maurer [7, 8] investigated the solubility of ammonia in aqueous salt-containing solutions. Feng et al. [9] and Schafer et al. [10, 11] reported the solubility data and the thermodynamic model for ammonia in methanol and in liquid mixture of water + methanol. Huang et al. [12] measured the solubility of ammonia in ethanol. Pan et al. [13] reported some solubility data of ammonia in mixed solvent of methanol + ethylene glycol. However, the solubility data of ammonia in ethylene glycol and the effect of pressure and temperature are still lacking in literature.
The aim of the presented work is to investigate the solubility of ammonia in ethylene glycol from (303.2 to 323.2) K and from ordinary pressure 0.101 MPa to low pressure 0.030 MPa. Furthermore, a thermodynamic model is developed based on the extended Raoult’s law and Henry’s law, correspondingstates correlations and molality scale based Pitzer’s equation to describe the phase equilibrium.
2 EXPERIMENTAL
2.1 Materials
Ammonia (mole fraction ≥0.995) was from Tianjin North Tianyi Chemical Company Limited (China) and was used without further purification. For the gas solubility measurements, ethylene glycol (mole fraction ≥0.998) purchased from Tianjin North Tianyi Chemical Company Limited (China) was chromatographically pure grade.
2.2 Apparatus
The solubility of ammonia in ethylene glycol wasmeasured by an isothermal solubility equilibrium method. The experimental apparatus is shown in Fig. 1. It consists of a 2 L glass vessel with a thermostated water jacket (Chemglass) and a stirrer (Heidolph RZR-2020). The temperature of the mixture inside the glass vessel was kept at the required temperature with a deviation of ±0.1 K using a thermostatic water bath (HUBER). The pressure inside the glass vessel was controlled to the preset value with a deviation of ±0.02 kPa by a vacuum pump (Vacuubrand ME4CNT), a vacuum controller (Vacuubrand CVC3000) and a 20 L pressure buffering bottle. The temperature and pressure were monitored by an online recorder. The ammonia was introduced into the glass vessel through a gas tube from the ammonia tank.
2.3 Procedure
The experimental procedure of isothermal solubility equilibrium method is as follows. (1) A volume of 1.0 L ethylene glycol was put into the glass vessel and heated to the preset temperature. (2) The system was stably kept at certain pressure by the vacuum pump and controller. (3) Ammonia was introduced into the solvent continuously, until the vapor-liquid equilibrium (VLE) was reached. (4) Three parallel samples were taken from the sampling valve.
The temperature of the mixture increases slightly while ammonia is dissolving in the solvent, and will stay at the preset temperature stably while the VLE is reached. A phenomenon also help us judge the equilibrium state, under which the gas tube does not suck the liquid when ammonia is stopped.
The ammonia concentration of the samples was determined by the chemical analysis as follows: (1) take and transfer the sample into a known amount (should be in excess) of hydrochloric acid solution to stabilize ammonia, (2) weigh the sample with a precision of 0.0001g, (3) titrate the excess hydrochloric acid by the standard solution of sodium hydrate to determine the amount of ammonia.
3 RESULTS AND DISCUSSION
3.1 Solubility of ammonia in ethylene glycol
The solubility of ammonia in ethylene glycol was measured at temperatures T=(303.2, 308.2, 313.2, 318.2, 323.2) K and total pressures p=(0.030, 0.040, 0.050, 0.060, 0.070, 0.080, 0.090, 0.100) MPa, in which ammonia molarity is in a range from 1.925 to 8.265 mol·kg−1. The experimental results of the solubility of ammonia in liquid ethylene glycol m2(mol·kg−1) are given in Table 1, where the corresponding concentration of ammonia in the vapor phase y2(mole fraction) is also listed. The experimental data and correlation results for the solubility of ammonia are plotted against total pressure at preset temperatures in Fig. 2.
3.2 Thermodynamic model of ammonia solubility
In vapor-liquid equilibrium, the fugacities of the solvent ethylene glycol and the solute ammonia accord with the extended Raoult’s law and Henry’s law respectively.
For the solvent ethylene glycol (component 1)

For the solute gas ammonia (component 2)
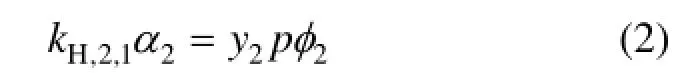

Figure 1 Experimental apparatus1—ammonia tank; 2,4—ammonia valve; 3—stirred glass vessel; 5—pressure buffering bottle; 6—pressure controller and vacuum pump; 7—tail gas absorbing equipment; 8—thermostatic water bath; 9—temperature and pressure online recorder; 10—sampling valve; 11—thermocouple thermometer; 12—gas tube; 13—pressure monitor

Table 1 Solubility and Henry’s law constant of ammonia in ethylene glycol
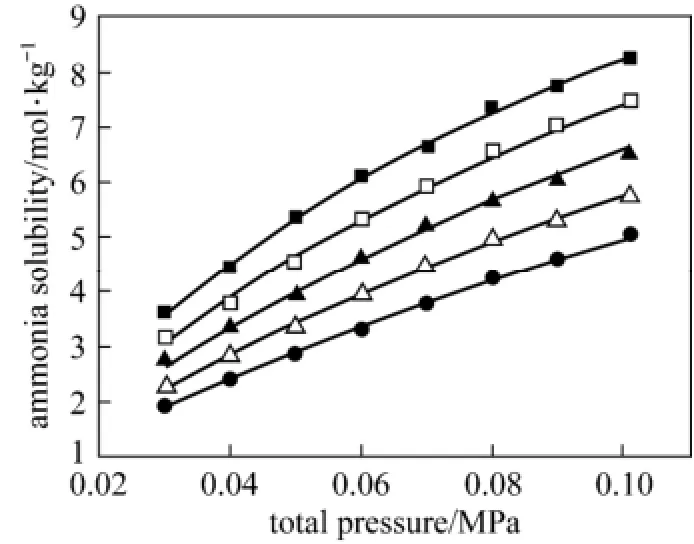
Figure 2 The molarity m2of ammonia in ethylene glycol plotted against the total pressure p for liquid mixtures of ammonia + ethylene glycol experimental data: ■ 303.2 K, □ 308.2 K, ▲ 313.2 K, △ 318.2 K,● 323.22 K;calculated data

There is a linear relationship between l n kH,2,1(p,T) and ( p−), whose intercept givesand the slope yields the partial molar volumes of ammonia in the liquid phaseis the Henry’s law constant (molality scale base) of ammonia in ethylene glycol at the saturation vapor pressure of pure ethylene glycol, which is expressed by the Benson and Krause equation [14].

where, δ2and T2are constants specific to the solute ammonia, and β is a universal constant. Since these parameters are unavailable in literature, they will be estimated from the ammonia solubility data.
The activity αiin Eqs. (1) and (2) is calculated by applying the molarity scale based Gibbs excess energy model of Pitzer [15, 16]. Following that model and neglecting chemical reactions in liquid mixtures of ammonia + ethylene glycol in the range investigated, for the gaseous solute ammonia, the activity is expressed as

where m0is the reference molality (m0=1 mol·kg−1), andand μ2,2,2(T) are binary and ternary parameters for interactions between ammonia molecules in the ethylene glycol.
For the solvent ethylene glycol, Pitzer’s model gives

The vapor pressure of pure ethylesne glycol can be calculated by Antoine equation l g p =a− b/(T +c) with the parameters listed in Table 2.
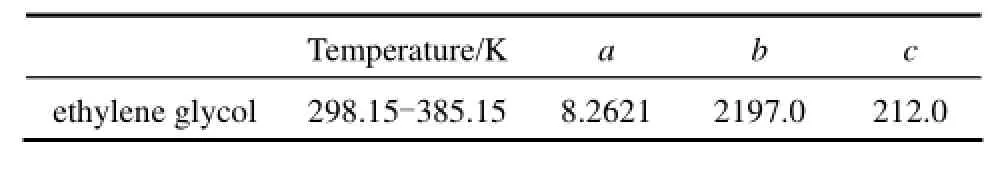
Table 2 Parameters in Antoine equation

where the second virial coefficient Biiof pure component is calculated from corresponding-state correlations. For the polar and hydrogen-bonded fluids, Tsonopoulos [14] gave a correlation for second virial coefficients in the following form,

where

where Tr=T/Tcis the reduced temperature. The parameters of critical temperature Tc, critical pressure pc, and acentric factor ω are listed in Table 3. Constants d1and d2cannot be easily generalized and are not available in literature, so they will be estimated from the ammonia solubility data.

Table 3 Acentric factor and critical parameters
The fugacity coefficients φiof ethylene glycol and ammonia in Eqs. (1) and (2) are calculated by the virial equation of state.
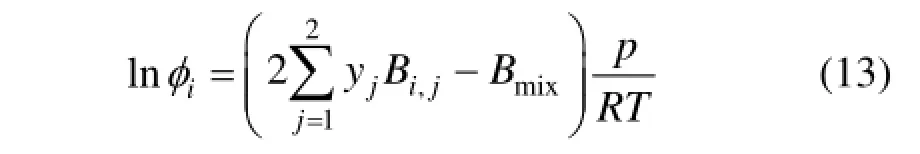
where the mixed second virial coefficient Bmixis a quadratic function of the mole fraction,

The cross-coefficient Bij(i≠j) can be calculated by Eqs. (9)-(12). And the common semiempirical combining rules are used to determine the specified parameters Tcij, Vcijand ωij, which are as follows.

3.3 Simulation results
The model requires (1) the model parameters d1and d2in Eq. (13),and μ2,2,2(T) in Eqs. (7) and (8), (2) physical propertiesandspecific constants δ2and T2of ammonia, and the universal constant β, and (3) the vapor phase composition yiHowever, these data are not available in literature, and yiwas not experimentally determined, so all properties/ parameters are determined from ammonia solubility data presented here, and the vapor phase composition is also estimated from the iteration process.
The model parameters d1and d2,and μ2,2,2(T) are set to zero as initial values and then estimated from the solubility data by genetic algorithm and least-squares method. The results are d1=0.01318, d2=0.00381, and a set of values ofand μ2,2,2(T) at different temperatures, which are correlated as follows.
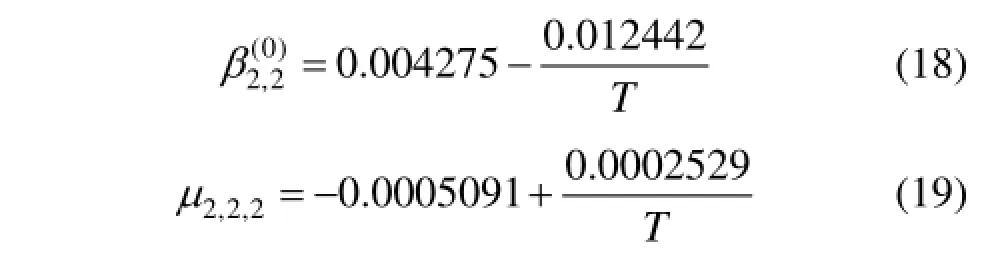

Figure 3 The influence of temperature and pressure on Henry’s law constant of ammonia in ethylene glycol experimental data: ■ 303.2 K, □ 308.2 K, ▲ 313.2 K, △318.2 K, ● 323.2 K;calculated data
Table 4 Values ofandbetween 303.2 and 323.2 K, 0.030 and 0.101 MPa

Table 4 Values ofandbetween 303.2 and 323.2 K, 0.030 and 0.101 MPa
T/K(0)H,exp/MPa k k ARD/% v2,1H,calc/MPa(0)∞/cm3·mol−1303.2 0.1302 0.1298 0.3072 21.1591 308.2 0.1470 0.1462 0.5442 20.5291 313.2 0.1645 0.1681 2.1884 20.5191 318.2 0.1995 0.1970 1.2531 17.9691 323.2 0.2349 0.2346 0.1277 16.6991

The ammonia specific constants δ2and T2and the universal constant β are determined from Eq. (4) with the values of, which gives δ2=17.927, T2= 356.16 K, and β=35.316.
Based on above model and parameters, the calculated values of Henry’s law constant kH,calc(p,T) from Eq. (3) are shown in Table 1 and Fig. 3, and the calculated values of Henry’s law constant at reference pressurefrom Eq. (4) are shown in Table 4 and Fig. 4. The average relative deviations (ARD) between the calculated and experimental data of kH(p,T ) andare 2.029% and 0.884% respectively. The calculated solubility of ammonia in ethylene glycol m2,calcis shown in Table 1 and Fig. 2, which has an overall ARD of 2.164% from the experimental result.
Figure 4 Logarithms of Henry’s law constant as a function of inverse temperature experimental data of this work;calculated data using Eq. (4)
4 CONCLUSIONS
The solubility of ammonia in liquid ethylene glycol was experimentally investigated. The measurements cover a temperature range from 303.2 K to 323.2 K under the negative pressure from 0.030 MPa to 0.101 MPa. The data were used to determine Henry’s law constant of ammonia in ethylene glycol and used in a thermodynamic model (based on the extended Raoult’s law and Henry’s law, corresponding-states correlations, and Pitzer’s molality scale based equation for the Gibbs excess energy) for the solubility of ammonia in ethylene glycol. The set of parameters reported here can be used to describe the solubility of ammonia in mixed solvent of ethylene glycol and methanol.
REFERENCES
1 Liang, Z. H., Li, S. Guo, W.Q., “The kinetics for electrochemical removal of ammonia in coking wastewater”, Chin. J. Chem. Eng., 19, 570-574 (2011).
2 Xuan, A.G., Wu, Y. X., Ma, P.S., “Measurement and correlation of solubility of carbon monoxide in phenol plus ethanol solvents”, Chin. J. Chem. Eng., 16, 762-765 (2008).
3 Amundsen, K., Eklund, H.R., Schmidt, R., “Process for producing anhydrous MgCl2”, US Pat., 6042794 (2000).
4 Zhou, H., Yuan, J.J., “Progress in preparation of high-purity anhydrous magnesium chloride”, Chinese J. Process Eng., 4, 276-281 (2004). (in Chinese)
5 Yan, Y., Lu X.C., Wang, T.Z., “Solubility of magnesium chloride hexammoniate in ethylene glycol solution saturated by ammonia gas”, J. Chem. Eng. Data, 55, 4827-4829 (2010).
6 Zhou, H., Hu, C.H., Yuan, J.J., “Technology of preparing high purity anhydrous magnesium chloride by glycol-ammonia method”, J. Salt and Chem. Industry, 36, 1-5 (2007). (in Chinese)
7 Rumpf, B., Maurer, G., “Solubility of ammonia in aqueous solutions of sodium sulfate and ammonium sulfate at temperatures from 333.15 K to 433.15 K and pressures up to 3 MPa”, Ind. Eng. Chem. Res., 32, 1780-1789 (1993).
8 Sing, R., Rumpf, B., Maurer, G., “Solubility of ammonia in aqueous solutions of single electrolytes sodium chloride, sodium nitrate, sodium acetate, and sodium hydroxide”, Ind. Eng. Chem. Res., 38, 2098-2109 (1999).
9 Feng, Y., Xie, R., Wu, Z., Marsh, K.N., “Vapor-liquid equilibria for ammonia + mehhanol”, J. Chem. Eng. Data, 44, 401-404 (1999).
10 Schafer, D., Xia, J., Vogt, M., Kamps, A.P.S., Maurer, G., “Experimental investigation of the solubility of ammonia in methanol”, Chem. Eng. Data, 52, 1653-1659 (2007).
11 Schafer, D., Vogt, M., Kamps, A.P.S., Maurer, G.., “Solubility of ammonia in liquid mixtures of (water + methanol)”, Fluid Phase Equilibria, 261, 306-312 (2007)
12 Huang, L.J., Xue, W.L., Zeng, Z.X., “The solubility of ammonia in ethanol between 227.35 K and 328.15 K”, Fluid Phase Equilibria, 303, 80-84 (2011).
13 Pan, L., Yang, Y.T., Zhou, H., Chen, Y.D., Zhang, S., “The solubility of ammonia in the mixed solvents of methanol and ethylene glycol”, Chem. Eng., 38, 48-53 (2010). (in Chinese)
14 Prausnitz, J.M., Molecular Thermodynamics of Fluid Phase Equilibria, 3rd edition, Printice Hall PTR, New Jersey, 165-168, 589, 600 (1999).
15 Pitzer, K.S., “Thermodynamics of electrolytes. 1. Theoretical basis andgeneral equations”, J. Phys. Chem., 77, 268-277 (1973).
16 Pitzer, K.S., “Ion interaction approach: theory and data correlation”, Activity Coefficients in Electrolyte Solutions, Pitzer, K.S., Ed., CRC Press, Boca Raton, FL, 75-155 (1991).
Received 2012-01-06, accepted 2012-09-10.
* Supported by the National Natural Science Foundation of China (21176189), and the Natural Science Foundation of Tianjin City (11JCZDJC24300).
** To whom correspondence should be addressed. E-mail: zhouhuan@tust.edu.cn
 Chinese Journal of Chemical Engineering2014年2期
Chinese Journal of Chemical Engineering2014年2期
- Chinese Journal of Chemical Engineering的其它文章
- Kinetics of Glucose Ethanolysis Catalyzed by Extremely Low Sulfuric Acid in Ethanol Medium*
- Synthesis of Sub-micrometer Lithium Iron Phosphate Particles for Lithium Ion Battery by Using Supercritical Hydrothermal Method
- Hydrogenation of Silicon Tetrachloride in Microwave Plasma
- Effects of Solvent and Impurities on Crystal Morphology of Zinc Lactate Trihydrate*
- Large-eddy Simulation of Ethanol Spray-Air Combustion and Its Experimental Validation*
- Kinetic and Thermodynamic Studies of Acid Scarlet 3R Adsorption onto Low-cost Adsorbent Developed from Sludge and Straw*
