Antisickling activity of butyl stearate isolated from Ocimum basilicum (Lamiaceae)
Dorothée Dinangayi Tshilanda, Pius Tshimankinda Mpiana*, Damase Nguwo Vele Onyamboko, Blaise Mavinga Mbala, Koto-te-Nyiwa Ngbolua, Damien Sha Tshibey Tshibangu, Matthieu Kokengo Bokolo, Kalulu Muzele Taba, Teddy Kabeya Kasonga
1Faculté des Sciences B.P. 190, Université de Kinshasa, Kinshasa XI, R.D. Congo
2Institut Supérieur Pédagogique de la Gombe (ISP-Gombe), Kinshasa, R.D. Congo
Antisickling activity of butyl stearate isolated from Ocimum basilicum (Lamiaceae)
Dorothée Dinangayi Tshilanda1, Pius Tshimankinda Mpiana1*, Damase Nguwo Vele Onyamboko1, Blaise Mavinga Mbala1, Koto-te-Nyiwa Ngbolua1, Damien Sha Tshibey Tshibangu1, Matthieu Kokengo Bokolo1, Kalulu Muzele Taba1, Teddy Kabeya Kasonga2
1Faculté des Sciences B.P. 190, Université de Kinshasa, Kinshasa XI, R.D. Congo
2Institut Supérieur Pédagogique de la Gombe (ISP-Gombe), Kinshasa, R.D. Congo
PEER REVIEW
Peer reviewer
Mr. André Mukala Nsengu Tshibangu, PhD; Associate Professor; Department of Basic Sciences, Faculty of Pharmaceutical Sciences, University of Kinshasa, P.O. Box 212; Kinshasa 11, Democratic Republic of the Congo.
Tel: +243(0)895104355
E-mail: andre28080@lycos.com
Comments
This is a valuable study in which the authors have isolate and characterized butyl stearate ester from O. basilicum as a new anti-sickling agent. The activity was assessed based on the sickling of red blood cells. Plant extracts and butyl stearate demonstrated remarkable sickling inhibitory effects. Treated sickle erythrocytes displayed a very similar phenotype/morphology than that of the normal erythrocytes one. O. basilicum is then promising source of anti-sickling new lead compounds.
Details on Page 397
Objective:To perform phytochemical analyses on the leaves of Ocimum basilicum L. (O. basilicum), to elucidate the structure of isolate and then perform the antisickling activity on the crude extract and on the isolate.
Sickle cell disease, Antisickling plant, Ocimum basilicum, Butyl stearate
1. Introduction
Sickle cell disease (SCD) or sickle cell anemia is a genetic disease that affects particularly the tropical areas. The disease is due to a replacement of glutamic acid located in the sixth position of the β chain of hemoglobin by valine[1,2].
This amino acid substitution alters not only the affinity of hemoglobin for oxygen but also its solubility in low oxygen pressure conditions. The decrease of solubility causespolymerization of hemoglobin and the sickling of red blood cells. This is the basis of many symptoms that sicklers suffer[1,3].
SCD affects each year nearly five million people in the world. In some parts of Africa, the sickle cell trait is up to 20% of the population with the prevalence in Central Africa of 25% to 30%[1]. More than 200 000 children with sickle cell disease are born each year in Africa[4].
Two percent of the populations, more than one million[5,6], in the Democratic Republic of the Congo (DRC) are affected by this disease. Nearly 80% of children affected by this anemia die before they are five years old if they are not followed medically[1,2,7,8].
The percentage of people suffering from this disease continues to grow. Therefore, sickle cell becomes a real public health problem in endemic areas[4]. It then becomes increasingly crucial to intensify early detection and to examine the search for new affordable treatments.
Several therapies have been proposed, the bone marrow transplantation, gene therapy, repeated blood transfusions, use of hydroxyurea,etc. But it turns out that these treatments are not only ineffective and very expensive for the poor African populations, but may also constitute a risk of HIV/AIDS[2,9,10].
Recently, several studies have dealt with the use of medicinal plants to treat SCD[11-14].
In DRC, recent findings have shown that at least 60 medicinal plants used in traditional medicine to treat SCD, among whichOcimum basilicum(O. basilicum), possesin vitroantisickling activity[5,6,9,15-19]. Those previous results showed that this activity could mainly be due to the presence of anthocyanins in the plants[2,5,6,15-17,20,21].
Some molecules isolated from plants, including p-hydroxy benzoic acid, Zanthoxylol, betulinic acid ... have shownin vitrointeresting activity against SCD[22,23]. Betulinic acid may thus be used as a positive control in antisickling activity assessment.
The present work is aimed to perform phytochemical analyses on the leaves ofO. basilicumand to elucidate the structure of the leaves bioactive compound.
2. Materials and methods
2.1. Plant material
O. basilicumleaves were harvested in the University of Kinshasa surroundings from May to June 2011. The plant was authenticated by Mr. Nlandu (Specimen voucher number 425) and deposited at the herbarium of Institut National des Recherches Agronomiques (INERA), the Faculty of Sciences of the University of Kinshasa.
2.2. Biological material
The homozygote HbS/HbS (SS) blood sample used to evaluate the biological activity was obtained from patients after their preventive treatment at the Centre de Médecine Mixte et d’Anémie SS, located in Kinshasa area, DRC. None of the patients had been transfused recently with homozygote HbA/HbA (AA) blood. All antisickling experiments were carried out with freshly collected blood. In order to confirm their SS nature, the above-mentioned blood samples were first characterized by haemoglobin electrophoresis on cellulose acetate gel at pH 8.5. They were found to be SS blood and were then stored in a refrigerator at 4 °C.
2.3. Extraction in acidified methanol
The dried and powdered plant material (leaves, 300 g) were soaked in 1 L of methanol and acidified with hydrochloric acid 0.4 mol/L and then concentrated to dryness under reduced pressure using a rotary evaporator. The residue was then extracted using petroleum ether and dried at 50 °C in the oven (Brand MEMMERT model).
Chemical screening was performed in aqueous and organic extracts according to a well known protocol.
2.4. Fractionation of the acidified methanolic extract
The thin layer chromatography with silica gel was carried out on the acidified methanolic extract using the mixture ofn-butanol-acetic acid-water (4-1-5) as eluting system and the UV lamp (type CAMAG) at 366 nm as a developer[24,25].
Preparative chromatography was performed on glass plates 20 cm×20 cm on which was spread P/UV254 silica gel. These plates were dried at 105 °C for 48 h in the oven.
Column chromatography was then carried out to isolate the mixtures from the preparative chromatography, using silica gel (Kieselgel brand 60 F254; 0.2-0.5 nm/35-70 100 mesh) and the mixture ofn-butanol/n-hexane (8:2) as eluting system.
2.5. Spectroscopic analyses
The structure elucidation of the compound isolated from the extract was done using 1D-NMR (1H-NMR,13C-NMR), 2DNMR (COSY, HMBC) and mass spectrum at high resolution.
NMR spectra were recorded using the spectrometer Bruker Avance 300 MHz type. All spectra were taken at roomtemperature using deuterated chloroform and read from the reference line deuterated chloroform which is δH7.24 ppm and δC77.20 ppm. The sample was solubilized in deuterated choloroform (CDCl3). The chemical shifts (δ) are expressed in ppm relative to tetramethylsilane.
The mass spectrometry was performed using the device“GCT Premier instrument”. The compound was dissolved in methanol (1:100) used for HPLC, and helium (0.8 mL/min) used as elution gas was maintained at 200 °C for 4 min and then programmed up to 300 °C, increasing the temperature by 5 °C a min. Ionization was done by electron impact and the analyzer was used high-resolution time of flight.
2.6. Antisickling activity
The blood sample was mixed with the crude extract and the isolate, using physiological saline as solvent. The Emmel test was performed to evaluate the antisickling activity[2]. Microscopic images were examined under an optical microscope brand Bresser Biolux NV 20X-1280X. Microscopic images were processed using the software
IMAGE MOTIC 2000 version 1.3.
3. Results
3.1 Antisickling activity of aqueous crude extracts of O. basilicum leaves
Figure 1, 2 and 3 provide digital images of respectively SS blood alone (negative control), of the SS blood treated with betulinic acid (positive control) and of that treated with acidified methanol extract.

Figure 1. Morphology of drepanocytes of SS blood (Negative control) [NaCl 0.9%, Na2S2O52% ×500].

Figure 2. Morphology of drepanocytes treated with betulinic acid (positive control) [NaCl 0.9%, Na2S2O52% ×500].

Figure 3. Morphology of drepanocytes treated with aqueous extract [NaCl 0.9%, Na2S2O52% ×500].
3.2 Phytochemical screening and extraction yield
The phytochemical screening on the crude extract revealed the presence of polyphenols (flavonoids, anthocyanins, leucoanthocyanins, tannins, quinones), alkaloids, saponins, triterpenoids and steroids.
The obtained extract after evaporation yielded 34.50 g (11.5%) out of 300 g of powdered leaves ofO. basilicum. The acidified methanolic extract showed an interesting antisickling activity.
3.3 Antisickling activity of acidified methanolic extract and the isolate
Figures 4 and 5 give the phenotype of SS red blood cells treated with the acidified methanolic extract and the isolate,respectively.
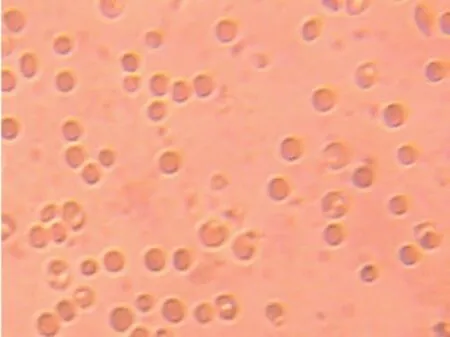
Figure 4. Morphology of drepanocytes treated with acidified methanolic extract [NaCl 0.9%, Na2S2O52% ×500].
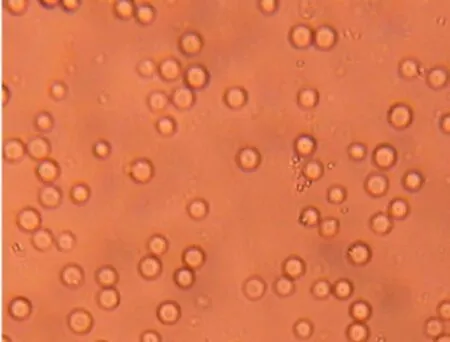
Figure 5. Morphology of drepanocytes with treated with the isolated compound [NaCl 0.9%, Na2S2O5 2% x500].
Calculated average of radius, perimeter and surface of drepanocytes before and after treatment with the isolated compound are given in Table 1.

Table 1 Average values of radius, perimeter and surface of erythrocytes before and after treatment the isolated compound (ICD) of O. basilicum.
3.4. Chromatographic analysis and spectroscopic characteristics of the isolate
Chromatographic analysis on the methanolic extract allowed to obtain atRf: 0.71, a viscous brown product, fluorescent at UV lamp at 366 nm, with a b.p. of 222-224 °C.
1H-NMR spectrum (CDCl3) showed characteristic peaks at δH: 4.064 ppm (t), 2.28 ppm (t); 0.93 ppm (t), 0.88 ppm (t) and 1.65 ppm to 1.31 ppm.
The13C-NMR spectrum showed 15 characteristic peaks at δC:174.0 ppm, 64.1 ppm, 34.4 ppm, 31.9 ppm, 30.7 ppm, 29.7 ppm, 29.6 ppm, 29.5 ppm, 29.3 ppm, 29.2 ppm, 25.0 ppm, 22.1 ppm, 19.1 ppm, 14.1 ppm, 13.7 ppm and a characteristic peak of great intensity at 29.7 ppm.
The mass spectrum, performed in electron impact ionization (EI) positive mode showed the molecular ion peak M+· at m/z: 340.334 5 and other peaks respectively at: 312.3, 257.2, 239.2, 213.18 and 199.17.
4. Discussion
4.1. Antisickling activity of O. basilicum leaves crude extracts
The majority of RBCs are elongated (sickled) confirming that the used blood is SS one. When betulinic (positive control) is added, RBCs show circular (biconcave) and normal shape. In the presence of the aqueous extract ofO. basilicum, the RBCs have circular (biconcave) and normal shape, which is similar to the positive control, indicating the antisickling effect of the extract. This confirms previous work of our research team[5,6,20] and justifies the use of this plant in Congolese folk medicine.
4.2. Phytochemical composition and Antisickling activity of acidified methanolic extract and that of the isolate.
Phytochemical screening ofO. basilicumleaves confirms previous works[18]. It was also indicates that polar extracts are more active on sickle blood cell that non polar one[2,11,15-18 ]. So antisickling activity of acidified methanolic extract was tested and fractioned. Bio guided tests were done on some fractions. Most of the red blood cells have recovered their normal and circular shapes under hypoxic conditions. This indicates the antisickling activity of the acidified methanolic extract and the isolate. These results can be quantified by calculating mean radius, perimeters and surfaces before and after RBCs treatment.
The used software could not calculate the average radius for the RBCs of the sickle blood since sickled RBCs of untreated blood are not circular. The average radius appeared after treatment of sickle RBCs with isolated compound indicating the re-appearance of the normal form of RBCs. Statistical treatment (Student test applied with a probability threshold of 0.05) enabled the determination of a significant difference between the average values of both the perimeter and the surface of blood cells on the micrography, thus confirming the modification RBCs morphology in the presence of the isolated compound. This behavior was already observed forsome extracts from other Congolese plants[2,7,8,18,19].
4.3. Structure elucidation of the isolate
The analysis of the1H-NMR spectrum indicates that characteristic peaks at δH: 4.064 ppm (t) and 2.28 ppm (t) represent the -CH2- groups respectively adjacent to the oxygen of the ester and to the carbonyl. The peaks at δH: 0.93 ppm (t) and 0.88 ppm (t) are characteristic of CH3 groups, respectively one that ends the methylene oxygen binder and the one ending those related to the carbonyl of the ester function, while those of δH: 1.65 ppm to 1.31 ppm represent the -CH2- groups between the long hydrocarbon chain methylene group linked to the carbonyl. These values are similar to those found in the literature[26].
In comparison of the literature[26] ,the 15 characteristic peaks of13C-NMR spectrum can be assigned as follows: C1 (δC 174.0 ppm), C2 (δC 64.1 ppm), C3 (δC 34.4 ppm ), C4 (δC 31.9 ppm), C5 (δC 30.7 ppm), C6 (δC 29.7 ppm) C7 (δC 29.6 ppm), C8 (δC 29.5 ppm), C9 (δC 29.3 ppm), C10 (δC 29.2 ppm) C11 (δC 25.0 ppm), C12 (δC 22.1 ppm), C13 (δC 19.1 ppm), C14 (δC 14.1 ppm), C15 (δC 13.7 ppm), and the characteristic peak of great intensity at δC 29.7ppm correspond to 8 methylene groups.
The mass spectrum gives an ion peak M+· at m/z: 340.3345 corresponding to butyl stearate mass. This correlates with the literature[26]. According to this literature, the compound was identified as butyl stearate.
This compound was synthesized and presented the same spectroscopic characteristics than the natural one.
O. basilicum, one of the plants used in Congolese traditional medicine against sickle cell anemia has shown antisickling activity and butyl stearate isolated from this plant could be the main active compound. To the best of our knowledge, this is the first time to report the antisickling activity of this compound in this plant. The synthesized compound presented the same spectroscopic characteristics than the natural one. Antisickling activities of its derivatives are understudying.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
The authors are indebted to the International Foundation for Science (IFS) and the Organization for the Prohibition of Chemical Weapons (OPCW) for the Research Grant given to one of them (Dr. Ngbolua Koto-Te-Nyiwa, IFS Research Grant F/4921-2) and to the University of Botswana and Dr .Oscar Mihigo Shetonde for performing spectral analyses.
Comments
Background
SCD is a blood disease characterized by the aggregation of hemoglobin S under hypoxic conditions. Its symptoms are erythrocytes shape modification and anemia. The need to search for new drugs with low toxicity is the new challenges. The plant kingdom could serve as a source of the antisickling new lead compounds as herein demonstrated.
Research frontiers
The present research work depicts in vitro anti-sickling activity and phytochemical analyses on the leaves ofO. basilicum. Biological testing was assessed by evaluating the effect of plant extracts and its isolate on the sickle erythrocytes phenotype/morphology. The structure of the isolate was elucidated using 1D-NMR (1H-NMR, 13C-NMR), 2D-NMR (COSY, HMBC) and mass spectroscopy at high resolution.
Related reports
There are several reports on the biological activities of this medicinal plant species as antimicrobial and antioxidant. This is the first time report on the anti-sickling activity of butyl stearate isolated fromO. basilicum.
Innovations and breakthroughs
O. basilicumleaves are widely used in the Congolese traditional medicine to treat SCD. In the present research study, the authors isolated for the first time butyl stearate fromO. basilicumand reported for the first time its antisickling activity.
Applications
The pharmaceutical relevance of findings from this study derives from the possibility of formulatingO. basilicumas an anti-sickling herbal medicine to be used in regions where SCD is endemic. The identification of the active principle could enhance the standardization of recipe.
Peer review
This is a valuable study in which the authors have isolate and characterized butyl stearate ester fromO. basilicumas a new anti-sickling agent. The activity was assessed based on the sickling of red blood cells. Plant extracts and butyl stearate demonstrated remarkable sickling inhibitory effects. Treated sickle erythrocytes displayed a very similar phenotype/morphology than that of the normal erythrocytesone.O. basilicumis then promising source of anti-sickling new lead compounds.
[1] Nelson DL, Cox MM. Lehninger principles of biochemistry. New York: WH Freeman; 2008, p. 75-189.
[2] Mpiana PT, Lombe BK, Ombeni AM, Ngbolua KTN, Tshibangu DST, Wimba LK, et al. In vitro sickling inhibitory effects and antisickle erythrocytes hemolysis of Dicliptera colorata CB Clarke, Euphorbia hirta L and Sorghum bicolor (L.) Moench. Open J Blood Dis 2013; 3: 43-48.
[3] Clark DP, Pazdernik NJ. Molecular biology. Waltham, USA: Elsevier Inc.; 2013, p. 417-457.
[4] World Health Organization. Sickle cell disease and other haemoglobin disorderss. Geneva: World Health Organization; 2011. [Online] Available from: http://www.who.int/mediacentre/ factsheets/fs308/en/. [Accessed on 20 June, 2013].
[5] Mpiana PT, Tshibangu DS, Shetonde OM, Ngbolua KN. In vitro antidrepanocytary activity (anti-sickle cell anemia) of some Congolese plants. Phytomedicine 2007; 14: 192-195.
[6] Mpiana PT, Mudogo V, Tshibangu DST, Shetonde OM, Ngbolua KN, Mbala MB, et al. In vitro antisickling activity of anthocyanins extract of a Congolese plant: Alchornea cordifilia. J Med Sci 2007; 7(7): 1182-1186.
[7] Mpiana PT, Dianzenza EN, Ngbolua KN, Mudogo V, Tshibangu DST, Mbala BM, et al. Antisickling properties, thermal and photochemical degradations of the anthocyanins extracted of Annona senegalensis from DR Congo. Int J Biol Chem Sci 2012; 6(5): 2241-2251.
[8] Mpiana PT, Bokota MT, Mbula JP, Ngbolua KN, Tshibangu DST, Atibu EK, et al. Effect of anthocyanin extracts from Justicia matammensis and Justicia laxa on sickle cells. In: Motashi N, editor. Anthocyanins: structure, biosynthesis and health benefits. New York: Nova publishers; 2012, p. 111-124
[9] Mpiana PT, Ngbolua KN, Mudogo V, Tshibangu DST, Atibu EK, Tshilanda DD, et al. Anti-sickle erythrocytes haemolysis properties and inhibitory effect of anthocyanins extracts of Trema orientalis (Ulmaceae) on the aggregation of human deoxyhemoglobin S in vitro. J Med Sci 2011; 11(3): 129-137
[10] Makani J, Ofori-Acquah SF, Nnodu O, Wonkam A, Ohene-Frempong K. Sickle cell disease: new opprtinuities and challenges in Africa. Scientific World Journal 2013; 2013: doi:10.1155/2013/193252.
[11] Ibrahim H, Sani FS, Danladi BH, Ahmadu AA. Phytochemical and antisickling studies of the leaves of Hymenocardia acida Tul (Euphorbiaceae), Pak J Biol Sci 2007; 10(5): 788-791.
[12] Okpuzor J, Adebesin O, Ogbunugafor H, Amadi I. The potential of medicinal plants in sickle celle disease control: a review. Int J Biomed Health Sci 2008; 4(2): 47-55.
[13] Sahu M, Singh V, Yadah S, Harris KK. Plant extracts with antisickling propensities: a feasible succor towards sickle cell disease management- a mini review. J Phytol 2012; 4(3): 24-29.
[14] Meselhy KM, Hammad LN, Farag N. Novel antisinckling, antioxidant and cytotoxic prenylated flavonoids from the barks of Morus alba L. Life Sci J 2012; 9(3): 830-841.
[15] Mpiana PT, Mudogo V, Tshibangu DS, Kitwa EK, Kanangila AB, Lumbu JB, et al. Antisickling activity of anthocyanins from Bombax pentadrum, Ficus capensis and Ziziphus mucronata: photodegradation effect. J Ethnopharmacol 2008; 120: 413-418.
[16] Mpiana PT, Mudogo V, Tshibangu DST, Ngbolua KN, Tshilanda DD, Atibu EK. Antisickling activity of anthocyanins of Jatropha curcas L. In: Singh VK, Govil JN, editors. Chemistry and medicinal value. Houston: Studium Press LLC; 2009, p. 83-90.
[17] Mpiana PT, Mudogo V, Ngbolua KN, Tshibangu DST, Atibu EK, Kitwa EK, et al. In vitro antisickling activity of anthocyanins extracts of Vigna unguiculata (L.) Walp. In: Singh VK, Govil JN, editors. Chemistry and medicinal value. Houston: Studium Press LLC; 2009, p. 75-82.
[18] Mpiana PT, Mudogo V, Tshibangu DST, Ngbolua KN, Manguala PK, Atibu EK, et al. Antisickling activity and thermodegradation of an anthocyanin fraction from Ocimum basilicum L. (Lamiaeceae). Comp Bio Nat Pro 2010; 3: 287-295.
[19] Mpiana PT, Makelele LK, Oleko RW, Bokota MT, Tshibangu DST, Ngbolua KN, et al. Antisickling activity of medicinal plants used in the management of sickle cell disease in Tshopo district, DR Congo. Aust J Med Herbal 2010; 22(4): 132-137.
[20] Mpiana PT, Mudogo V, Tshibangu DST, Shetonde OM, Ngbolua KN, Mbala MB. Antisickling activity of some congolese plants. In: Drug discovery from African flora. The 12th symposium of the natural product research network for Eastern and Central Africa; 2007 Jul 22-26; Kampala, Uganda. 2007. p. 45.
[21] Mpiana PT, Mudogo V, Kabangu YF, Tshibangu DST, Ngbolua KN, Atibu EK, et al. Antisickling activity and thermostability of anthocyanins extract from a Congolese plant, Hymenocardia acida Tul. (Hymenocardiaceae). Int J Pharmacol 2009; 5(1): 65-70.
[22] Sofowora EA, Isaac-Sodeye WA, Ogunkoya LO. Isolation and charactesation of an antisickling agent from Fagara zanthoxylloide root. Lioyidia 1975; 38: 169-171.
[23] Tshibangu DST. Phytochemical and anti-drepanocytosis studies of Cajanus cajan, Callistemon viminalis, Melaleuca bracteata Var, revolution gold and Syzygium guineense. Westville, Durban: University of Kwazulu- Natal; 2010.
[24] Wixon RL, Gehrke CW, editors. Chromatography: a science of discovey. New Jersey: John Wiley and Sons, Inc.; 2011, p. 61-100.
[25] Dash DC. Analytical chemistry. New Delhi: PHI Learning Pvt. Ltd.; 2011, p. 213-262.
[26] National Institute of Advanced Industrial Science and Technology (AIST). Spectral database for organic compounds SDBS. Japan: AIST. [Online] Available from: http://sdbs.db.aist.go.jp/sdbs/cgibin/cre_index.cgi. [Accessed on 20 June, 2013].
10.12980/APJTB.4.2014C1329
*Corresponding author: Prof. Dr. Pius T. Mpiana, PhD, Département de Chimie, Faculté des Sciences, Université de Kinshasa, B.P 190 Kinshasa XI, Democratic Republic of the Congo.
Tel : +243 81811 6019
E-mail : ptmpiana@yahoo.fr
Foundation Project: Supported by the International Foundation for Science (IFS) and the Organization for the Prohibition of Chemical Weapons (OPCW) for the Research Grant (IFS Research Grant F/4921-2).
Article history:
Received 4 Feb 2013
Received in revised form 12 Feb, 2nd revised form 19 Feb, 3rd revised form 6 Mar 2014
Accepted 20 Apr 2014
Available online 28 May 2014
Methods:The Emmel test performed on the acidified methanolic extract of this plant was used to evaluate the antisickling activity. The structure characterization of the active compound was performed using chromatographic techniques for the separation and the spectroscopic ones for structure elucidation (1H-NMR, 13C-NMR, COSY, HMBC).
Results:The chemical screening on the crude extract revealed the presence of polyphenols (flavonoids, anthocyanins, leucoanthocyanins, tannins, quinones) alkaloids, saponins, triterpenoids and steroids. The obtained extract after evaporation yielded 34.50 g (11.5%) out of 300 g of powdered leaves of O. basilicum. The acidified methanolic extract and butyl stearate showed an interesting antisickling activity.
Conclusions:The acidified methanolic extract and butyl stearate from O. basilicum displayed a good antisickling activity. To the best of our knowledge, this is the first time to report the antisickling activity of this compound in this plant. The synthesized compound presented the same spectroscopic characteristics than the natural one and the antisickling activities of its derivatives are understudying.
 Asian Pacific Journal of Tropical Biomedicine2014年5期
Asian Pacific Journal of Tropical Biomedicine2014年5期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Ethnobotanical survey of folklore plants used in treatment of snakebite in Paschim Medinipur district, West Bengal
- Pharmacognostic studies of stem, roots and leaves of Malva parviflora L.
- Rapid detection of coliforms in drinking water of Arak city using multiplex PCR method in comparison with the standard method of culture (Most Probably Number)
- Salvia fruticosa reduces intrinsic cellular and H2O2-induced DNA oxidation in HEK 293 cells; assessment using flow cytometry
- Antioxidant and antimicrobial properties of Litsea elliptica Blume and Litsea resinosa Blume (Lauraceae)
- Tamarind seed coat extract restores reactive oxygen species through attenuation of glutathione level and antioxidant enzyme expression in human skin fibroblasts in response to oxidative stress
