Phytochemical screening and anticonvulsant studies of ethyl acetate fraction of Globimetula braunii on laboratory animals
Musa Mumammad Aliyu, Abdullahi Isma’il Musa, Muhammad Ja’afar Kamal, Magaji Garba Mohammed
1Department of Pharmaceutical and Medicinal Chemistry, Ahmadu Bello University, Zaria-Nigeria
2Department of Pharmaceutical and Medicinal Chemistry, Usmanu Danfodio University, Sokoto-Nigeria
3Chemistry Advance Laboratory, Sheda Science and Technology Complex, Sheda- Abuja-Nigeria
4Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria-Nigeria
Phytochemical screening and anticonvulsant studies of ethyl acetate fraction of Globimetula braunii on laboratory animals
Musa Mumammad Aliyu1, Abdullahi Isma’il Musa2, Muhammad Ja’afar Kamal3, Magaji Garba Mohammed4*
1Department of Pharmaceutical and Medicinal Chemistry, Ahmadu Bello University, Zaria-Nigeria
2Department of Pharmaceutical and Medicinal Chemistry, Usmanu Danfodio University, Sokoto-Nigeria
3Chemistry Advance Laboratory, Sheda Science and Technology Complex, Sheda- Abuja-Nigeria
4Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria-Nigeria
PEER REVIEW
Peer reviewer
Prof MJ Potgieter, University of Limpopo, South Africa.
Tel: +27 15 268 2224
E-mail: Martin.potgieter@ul.ac.za
Comments
This is a valuable research work in which authors have demonstrated the phytochemical properties and anticonvulsant activities of EAF of G. braunii on laboratory animals. The activity was assessed based on anticonvulsant activity using maximal electroshock test in chicks, as well as pentylenetetrazole and 4-AP-induced seizures in mice. The preliminary phytochemical screening carried out on the crude ethanol extract revealed the presence of saponins, carbohydrates, flavonoids, tannins, anthraquinones and steroids. Similarly, tannins, flavonoids and steroids/terpenes were found to be present in the EAF.
Details on Page 288
Objective:To investigate the phytochemical properties and the anticonvulsant potential of the ethyl acetate soluble fraction of ethanol leaf extract of Globimetula braunii, a plant used in ethnomedicine for the treatment of epilepsy.
Epilepsy, Globimetula braunii, Seizure, Medicinal, Pentylenetetrazole
1. Introduction
Epilepsy is a common and diverse set of chronic neurological disorders characterized by seizures[1]. About 75%-80% of epileptic patients may be provided with adequate seizure control with the help of conventional antiepileptic drugs. However, over 30% of people with epilepsy do not have seizures control even with best available medications[2]. Currently available antiepileptic drugs do not affect epileptogenesis and are associated with serious side effects,including teratogenicity, chronic toxicity and adverse effects on cognition and behavior[3]. Almost all the currently available antiepileptic drugs are associated with drug interaction making it difficult to attain easy seizure control[4]. There is an urgent need for the development of newer antiepileptic agents with better safety and efficacy profile. There is a reawakening interest in traditional medicine in the management of epilepsy, especially in developing countries[5]. Researches are needed to validate the folkloric use of these medicinal plants in order to provide evidence of their safety and efficacy[6]. One of such medicinal plants used in the traditional management of epilepsy but with paucity of scientific verification literature isGlobimetula braunii(G. braunii) (Mistletoe).
G. braunii(Engler) Van Tlegh is a member of the Loranthaceae family, a hemi-parasitic shrub that grows on dicotyledones trees and attached itself to the host by modified roots. The vernacular names in Nigeria include“Kauchii” (Hausa) and “Afomoonishano” (Yoruba). The leaves ofG. brauniiare used for the treatment of disease like cardiovascular diseases[7], hepatic illness[8,9] and malaria[10]. It also has common application in treating rheumatism, epilepsy, infertility, stomach problems, and as a laxative[11]. To the best of our search, there is no report of the anticonvulsant activity ofG. brauniiin the literature. In furtherance with an attempt to isolate the bioactive principles of the plant, this study therefore aimed at establishing the phytochemical constituent present and evaluating the anticonvulsant potential of the ethyl acetate fraction ofG. braunii.
2. Materials and methods
2.1. Materials
2.1.1. Collection and identification of plant material
The leaves ofG. brauniiparasitic onPiliostigma thonningiiwere collected in October 2011, at the Botanical garden of medicinal plants of Sheda science and Technology complex (Shestco), Abuja, Nigeria. This was confirmed and authenticated at the herbarium, Biological Sciences Department, Ahmadu Bello University, Zaria, Nigeria, by comparing with existing specimens (No. 9016 forG. brauniiand No. 7151 forPiliostigma thonningii).
2.1.2. Preparation of extract
The plant leaves were washed, air dried under the shade until constant weight was obtained, size was reduced using laboratory blender and subsequently referred to as powdered plant material. The powdered plant material (250 g) was subjected to extraction with ethanol in a Soxhlet apparatus for 48 h. The Solvent was removed in vacuo to yield a residue (80 g) referred to asG. brauniiethanolic extract. The ethanol extract (70 g) was suspended in distilled water and partitioned successively withn-hexane, chloroform, ethyl acetate andn-butanol to obtainn-hexane fraction, chloroform fraction, ethyl acetate fraction (EAF),n-butanol fraction and the aqueous fraction respectively.
2.1.3. Animals
Locally bred adult Swiss albino mice of either sex weighing (20±2) g were obtained from the Animal House Facility of the Department of Pharmacology and Therapeutics, Ahmadu Bello University Zaria, Nigeria. Day old ranger cockerels (30±5) g were obtained from Zarm farms Kwara state, Nigeria. The mice, maintained on standard rodent faced and waterad libitum, were housed in polypropylene cages at room temperature throughout the study.
2.2. Methodology
2.2.1. Phytochemical screening
Portion of the ethanol extract and that of EAF ofG. brauniiwere subjected to preliminary phytochemical screening using standard methods[12].
2.2.2. Acute toxicity studies in mice
The method used was described by Lorke[13]. All mice were injected intra peritoneally (i.p). The study was divided into two phase. In the first phase, nine mice of either sex were divided into groups of three mice each. Group one received 10 mg/kg extract while groups two and three received 100 mg/kg and 1 000 mg/kg respectively. The animals were observed for signs and symptoms of toxicity including mortality for forty eight hours after treatment. In the second phase, four mice of either sex were divided into four groups of one mouse each. The first mouse received extract at a dose of 600 mg/kg while the second, third and fourth received the extract at doses of 1 000 mg/kg, 1 600 mg/kg and 2 900 mg/kg respectively, based on the outcome of the first phase. The mice were also observed for twenty four hours. The final LD50was estimated as the square root of the products of the lowest lethal dose and the highest non-lethal dosei.e.geometric mean of consecutive doses for which 0% and 100% survival rates were recorded.
2.2.3. Maximal electroshock seizures test in chicks
Day old ranger cockerels (fifty in number) were randomly divided into five groups of ten chicks each. The first group received normal saline (10 mL/kg)i.p, second, third and fourth groups were administered 75 mg/kg, 150 mg/kg and 300 mg/kgi.pof EAF respectively, and the fifth group was administered 20 mg/kg of phenytoin. Thirty minutes after treatment, maximal electroshock was administered to induce seizures in the chicks using Ugo Basile electroconvulsive machine (Model 7801) with comeal electrodes placed on the upper eyelids of the chicks. The shock duration, frequency and pulse width were set and maintained at 0.8 seconds, 100 pulses/seconds and 0.8 m/s respectively. A current of 70 mA which produced tonic seizures in 80% of the controlchicks, were used throughout the study. An episode of tonic extension of the hind limbs was regarded as full convulsion, while lack of tonic extension of the hind limbs was regarded as protection. In unprotected animals, the recovery time was recorded[14].

Table 1 Results of phytochemical analysis of ethyl acetate fraction of G. braunii leaves.
2.2.4. Pentylenetetrazole induced seizure in mice (PTZ)
Thirty six mice were randomly divided into six groups of six mice each. Group one (negative control group) was given 10 mL/kgi.pof normal saline, group two, three and four were given 75 mg/kg, 150 mg/kg and 300 mg/kgi.pof EAF respectively. Fifth group (positive control) received 200 mg/kgi.psodium valproate. Thirty minutes after treatment, 90 mg/ kg of freshly prepared solution of PTZ (CD97) was administered to each mouse, subcutaneously. The mice were observed for thirty minutes for presence or absence of threshold seizures (i.e.an episode of clonic spasm of at least five seconds duration)[15].
2.2.5. 4-Aminopyridine-induced seizures test (4-AP)
The method previously described by Yamaguchi and Rogawski was adopted[16]. Thirty six albino mice were randomly divided into six groups of six mice each. Group one (negative control) was administered with 10 mL/kg of normal saline while groups two, three and four received EAF (75, 150 and 300 mg/kg),i.p. The fifth group received phenobarbitone (20 mg/kg). Thirty minutes after treatment, 13.5 mg/kg of freshly prepared 4-AP was administered subcutaneously to each mouse. Ability of the extract to protect the mice from lethality within a thirty minutes observation period was considered an indication of anticonvulsant activity[17].
3. Results
The preliminary phytochemical screening revealed the presence of saponins, carbohydrates flavonoids, cardiac glycoside, anthraquinones and steroids in the crude ethanol extract while the ethyl acetate fraction was found to contain tannins, flavonoids and steroids/terpenes (Table 1).
All the control animals exhibited seizures after delivery of electroshock. The extract did not protect the animals against tonic limbs extension at all the tested doses (Table 2).
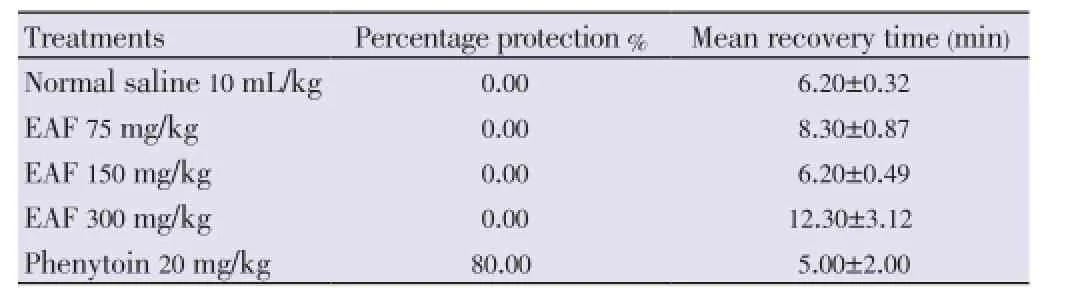
Table 2 Effect of EAF against maximal electroshock in chicks.
The entire control animal exhibited myoclonic jerk and some exhibited threshold seizures and loss of righting reflex with tonic forelimbs extension. The extract exhibited anticonvulsant effect on seizure included by subcutaneous PTZ. It offered maximum protection against threshold seizure with 88.33% at a dose of 150 mg/kg (Table 3).

Table 3 Effect of EAF against pentylenetrazole induced seizure.
The extract produced a reasonable protection against 4-AP induced seizure compared to the control. However, the extract produced an increase in the mean onset of seizure in unprotected animals (Table 4).
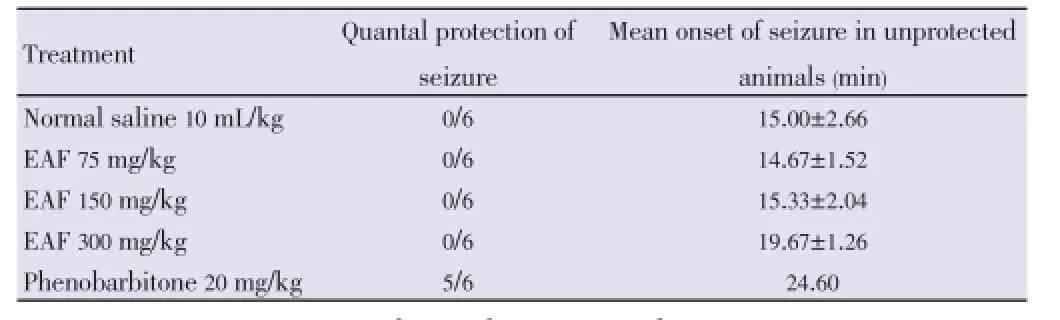
Table 4 Effect of EAF against subcutaneous 4-AP induced seizure.
4. Discussion
The result of preliminary phytochemical screening carried out on the crude ethanol extract revealed the presence of saponins, carbohydrates flavonoids, cardiac glycoside, anthraquinones and steroids, while that of the ethyl acetate fraction revealed the presence of tannins, flavonoids and steroids/terpenes. These phytochemical constituents have been reported to be associated with different pharmacological activities of plants[18]. Triterpenes and steroids, among other phytochemicals have been reported to possess anticonvulsant activity[19].
The data presented in this study provide scientific evidence that EAF obtained from the crude ethanol extract ofG. brauniileaves may contain psychoactive principles that are relevant to the management of convulsive disorder. The intraperitoneal median lethal dose of EAF found to be 1 261.91 mg/kg in mice suggests that it is relatively toxic[20]. However, it is relatively safe at the dose employed in this study.
Maximal electroshock seizure can be prevented by sodium channel blockers such as phenytoin, valproate, felbamate and lamotrigine[21] or agents that block glutamatergic neurotransmission mediated by n-methyl-d-aspartate (NMDA) receptors[22]. Agents which protect animal against MEST have been shown to be beneficial in the management of generalized tonic clonic seizure. Therefore, the absence of anticonvulsant ability in MEST suggests that EAF may not be useful in the treatment of generalized tonic clonic and partial seizures.
EAF protected mice against pentylenetetrazole and significantly delayed the onset of myoclonus jerks and tonic seizures. It is widely accepted that PTZ causes seizures by blocking the major GABAergic inhibitory pathways[23]. Standard antiepileptic drugs such as diazepam and phenobarbitone are thought to produce their effects by enhancing GABA mediated inhibition in the brain[24]. Seizures induced by PTZ can also be blocked by drugs such as ethosuximide by reducing T-type Ca2+currents[25].
Activation of the NMDA receptor system is also involved in the initiation and propagation of PTZ-induced seizures[26]. In this regard, drugs such as felbamate that reduces glutamate release by blocking presynaptic NMDA receptors in the entorhinal cortex have demonstrated anticonvulsant activity against PTZ induced seizures[27]. It is therefore possible that the anticonvulsant effect shown in this study by EAF against seizures produced by PTZ might be due to enhancement of GABAergic neurotransmission, inhibition of T-type Ca2+current or blockade of glutamateergic neuro transmission mediated by NMDA receptor, which are not tested in this study.
K+channels play a significant role in controlling all aspect of neuronal excitability[28]. Sodium channel blockers, such as phenytoin which prevent seizure spread effectively antagonize seizures induced by K+channel blocker such as 4-AP while those with specific actions on other cellular targets may be weak or inactive, presumably because they are unable to attenuate the spread of intense (non-NMDA receptor mediated) excitation evoked by 4-AP. EAF failed to abolish the spontaneous discharges induced by 4-AP in mice. The inability of the fractions to produce significant activities against 4-AP induced seizure suggests that they may likely not be interacting with K+channel in producing their anticonvulsant activities.
The findings of this study suggest that the EAF ofG. brauniileaves extract possesses psychoactive principles that may be useful in the management of petit mal epilepsy and lend credence to the ethnomedical use of the plant in the management of epilepsy.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgements
This research was supported by Ahmadu Bello University Board of Research Grant (Grant No.:DAPM/BOD/05).
Comments
Background
The ethyl acetate soluble fraction of ethanol leaf extract ofG. brauniiused in ethnomedicine for the treatment of epilepsy and diabetes among other ailments, was subjected to phytochemical and anticonvulsant screening.
Research frontiers
The present research work presents a report of the anticonvulsant activity ofG. braunii, as no information could be found in the literature. In furtherance with an attempt to isolate the bioactive principles of the plant, this study aimed at establishing the phytochemical constituent present, and evaluating the anticonvulsant potential of the EAF ofG. braunii.
Related reports
The leaves ofG. brauniiare used for the treatment of ailments like cardiovascular diseases (Ouedraogoet al.,2004), hepatic illness (Phillipson and Wright, 1991; Olagunjuet al., 1999; Al-Ghaithiet al., 2004) and malaria (Traoreet al., 2000).
Innovations and breakthroughs
These results suggest that the EAF extract ofG. brauniileaves possesses psychoactive compounds.
Applications
These results may be useful in the management of petit mal epilepsy and lend credence to the ethnomedical use of the plant in the management of epilepsy.
Peer review
This is a valuable research work in which authors have demonstrated the phytochemical properties and anticonvulsant activities of EAF ofG. brauniion laboratory animals. The activity was assessed based on anticonvulsant activity using maximal electroshock test in chicks, as well as pentylenetetrazole and 4-AP-induced seizures in mice. The preliminary phytochemical screening carried out on the crude ethanol extract revealed the presence of saponins, carbohydrates, flavonoids, tannins, anthraquinones and steroids. Similarly, tannins, flavonoids and steroids/terpenes were found to be present in the EAF.
[1] Nath PS, Meena YK, Laxmi T. Synthesis and anticonvulsant activity (chemo shock) of phenothiazine amino acid derivatives. Chem Sci Trans 2013; 2(1): 123-128.
[2] Yemitan OK, Adeyemi OO. Antiepileptogenic and anticonvulsant actions of Dalbergia saxatilis (Hook, F.) in sub-toxic chemical kindling and toxic convulsant models. Eur J Med Plants 2013; 3(2): 288-296.
[3] Johannessen Landmark C, Patsalos PN. Drug interactions involving the new second- and third-generation antiepileptic drugs. Expert Rev Neurother 2010; 10 (1): 119-140.
[4] Hela SI, Megahed HS, Salem SM, Youness ER. Monotherapy versus polytherapy in epileptic adolescents. Maced J Med Sci 2013; 6(2):174-177.
[5] Magaji MG, Yaro AH, Musa AM, Anuka JA, Abdu-Aguye I, Hussaini IM. Central depressant activity of butanol fraction of Securinega virosa root bark in mice. J Ethnopharmacol 2012; 141: 128-133.
[6] Maiha BB, Magaji MG, Yaro AH, Ahmed ST, Hamza AH, Magaji RA. Anticonvulsant studies on Cochlospermum tinctorium and Paullinia pinnata extracts in laboratory animals. Niger J Pharm Sci 2009; 8(1): 102-108.
[7] Ouédraogo S, Ralay Ranaivo H, Ndiaye M, Kaboré ZI, Guissou IP, Bucher B, et al. Cardiovascular properties of aqueous extract from Mitragyna inermis (wild). J Ethnopharmacol 2004; 93: 345-350.
[8] Phillipson JD, Wright CW. Can ethnopharmacology contribute to the development of antimalarial agents. J Ethnopharmacol 1991; 32: 155-165.
[9] Al-Ghaithi F, El-Ridi MR, Adeghate E, Amiri MH. Biochemical effect of Citrullus colocynthis in normal and diabetic rats. Mol Cell Biochem 2004; 261: 143-149.
[10] Traore F, Gasquet M, Laget M, Guiraud H, Di Giorgio C, Azas N, et al. Toxicity and genotoxicity of antimalarial alkaloid rich extracts derived from Mitragyma inermis O. Kuntze and Nuclea latifolia. Phytother Res 2000; 4: 608-611.
[11] Olagunju JA, Ismail FI, Gbile ZO. The hypoglycaemic property of normal saline leaf of Globimetula braunii in alloxanised diabetic albino rats. Biomed lett 1999; 60(235): 83-89.
[12] Trease GE, Evans WC. A textbook of pharmacology. 9th ed. London: Balliere, Tindall & Cassell; 1966.
[13] Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol 1983; 54: 275-289.
[14] Sayyah M, Valizadeh J, Kamalinejad M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole and maximal electroshock-induced seizures. Phytomedicine 2002; 9: 212-216.
[15] Swinyard EA. Laboratory evaluation of antiepileptic drugs. Review of laboratory methods. Epilepsia 1989; 10: 107-119.
[16] Yamaguchi S, Rogawski MA. Effects of anticonvulsant drugs on 4-aminopyridine-induced seizures in mice. Epilepsy Res 1992; 11: 9-16.
[17] Ayanwuyi LO, Yaro AH, Adamu HYS. Studies on anticonvulsant activity of methanol capitulum extract of Leonatis nepetifolia Linn. Niger J Pharm Sci 2009; 8(1): 73-79.
[18] Turker AU, Usta C. Biological screening of some Turkish medicinal plants for antimicrobial and toxicity studies. Nat Prod Res 2008; 22: 136-146.
[19] Barua CC, Begum SA, Barua AG, Borah RS, Lahkar M. Anxiolytic and anticonvulsant activity of methanol extract of leaves of Alternanthera brasiliana (L.) Kuntze (Amaranthaceae) in laboratory animals. Indian J Exp Biol 2013; 51(6): 450-457.
[20] Matsumura F. Toxicology of Insecticides. 2nd ed. New York; Plenum Press; 1985, p. 588.
[21] Sayyah M, Nadjafnia L, Kamalinejad M. Anticonvulsant activity and chemical composition of Artemisia dracunculus L. essential oil. J Ethnopharmacol 2004; 94: 283-287.
[22] Subramaniam S, Rho JM, Penix L, Donevan SD, Fielding RP, Rogawski MA. Felbamate block of the N-methyl-D-aspartate receptor. J Pharmacol Exp Ther 1995; 273(2): 878-886.
[23] Prigol M, Brüning CA, Godoi B, Nogueira CW, Zeni G. m-Trifluoromethyl-diphenyl diselenide attenuates pentylenetetrazole-induced seizures in mice byinhibiting GABA uptake in cerebral cortex slices. Pharmacol Rep 2009; 61: 1127-1133.
[24] Czapin´ski P, Blaszczyk B, Czuczwar SJ. Mechanisms of action of antiepileptic drugs. Curr Top Med Chem 2005; 5: 3-14.
[25] Sun XY, Zhang L, Wei CX, Piao HR, Quan ZS. Characterization of the anticonvulsant activity of doxepin in various experimental seizure models in mice. Pharmacol Rep 2009; 61: 245-251.
[26] Yudkoff M, Daikhin Y, Nissim I, Horyn O, Luhovyy B, Lazarow A, et al. Short-term fasting, seizure control and brain amino acid metabolism. Neurochem Int 2006; 48: 650-656.
[27] Yang J, Wetterstrand C, Jones RS. Felbamate but not phenytoin or gabapentin reduces glutamate release by blocking presynaptic NMDA receptors in the entorhinal cortex. Epilepsy Res 2007; 77 (2-3): 157-164.
[28] Wickenden AD. Potassium channels as antiepileptic drug targets. Neuropharmacology 2002; 43: 1055-1060.
10.12980/APJTB.4.2014C925
*Corresponding author: Mohammed Garba Magaji, PhD; Department of Pharmacology and Therapeutics, Ahmadu Bello University, Zaria-Nigeria.
Tel: +2348034685849
E-mail: mgmagaji@abu.edu.ng
Foundation Project: This research was supported by Ahmadu Bello University Board of Research Grant (Grant No.:DAPM/BOD/05).
Article history:
Received 9 Feb 2014
Received in revised form 13 Feb, 2nd revised form 18 Feb, 3rd revised form 22 Feb 2014
Accepted 24 Apr 2014
Available online 28 Apr 2014
Methods:The phytochemical screening was carried out using standard protocol while the anticonvulsant activity was studied using maximal electroshock test in chicks, pentylenetetrazole and 4-aminopyridine-induced seizures in mice.
Results:The preliminary phytochemical screening carried out on the crude ethanol extract revealed the presence of saponins, carbohydrates, flavonoids, tannins, anthraquinones and steroids. Similarly, tannins, flavonoids and steroids/terpenes were found to be present in the ethyl acetate fraction. In the pharmacological screening, 150 mg/kg of the fraction protected 83.33% of animals against pentylenetetrazole-induced seizure in mice whereas sodium valproate a standard anti-epileptic drug offered 100% protection. In the 4-aminopyridine-induced seizure model, the fraction produced a significant (P<0.05) increase in the mean onset of seizure in unprotected animals. The fraction did not exhibit a significant activity against maximal electroshock convulsion. The median lethal dose of the fraction was found to be 1 261.91 mg/kg.
Conclusions:These results suggest that the ethyl acetate fraction of Globimetula braunii leaves extract possesses psychoactive compound that may be useful in the management of petit mal epilepsy and lend credence to the ethnomedical use of the plant in the management of epilepsy.
 Asian Pacific Journal of Tropical Biomedicine2014年4期
Asian Pacific Journal of Tropical Biomedicine2014年4期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- The effects of exposure to pesticides on the fecundity status of farm workers resident in a rural region of Fars province, southern Iran
- A cross sectional study on antibiotic resistance pattern of Salmonella typhi clinical isolates from Bangladesh
- Comparative susceptibility to permethrin of two Anopheles gambiae s.l. populations from Southern Benin, regarding mosquito sex, physiological status, and mosquito age
- Effects of melatonin on changes in cognitive performances and brain malondialdehyde concentration induced by sub-chronic coadministration of chlorpyrifos and cypermethrin in male Wister rats
- Phytochemical and in vitro biological investigations of methanolic extracts of Enhydra fluctuans Lour.
- Theoretical and experimental study on lipophilicity and wound healing activity of ginger compounds
