Study of the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions
Nazaire Aïzoun, Roseric Azondekon, Rock Aïkpon, Virgile Gnanguenon, Razaki Osse, Alex Asidi, Martin Akogbéto
1Centre de Recherche Entomologique de Cotonou (CREC), 06 BP 2604, Cotonou, Bénin
2Faculté des Sciences et Techniques, Université d'Abomey Calavi, Calavi, Bénin
3University of Massachusetts Amherst, Amherst, Massachusetts, USA
4London School of Hygiene and Tropical Medecine, Keppel Street WC1E 7HT, United Kingdom
Study of the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions
Nazaire Aïzoun1,2*, Roseric Azondekon1,3, Rock Aïkpon1,2, Virgile Gnanguenon1,2, Razaki Osse1,2, Alex Asidi4, Martin Akogbéto1,2
1Centre de Recherche Entomologique de Cotonou (CREC), 06 BP 2604, Cotonou, Bénin
2Faculté des Sciences et Techniques, Université d'Abomey Calavi, Calavi, Bénin
3University of Massachusetts Amherst, Amherst, Massachusetts, USA
4London School of Hygiene and Tropical Medecine, Keppel Street WC1E 7HT, United Kingdom
PEER REVIEW
Peer reviewer
Dr. Padonou Germain Gil, Medical entomologist, Faculté des Sciences et Techniques, UAC, Benin.
Tel: 00229 97289615
E-mail: pagergil@yahoo.fr
Comments
This is a valuable research work in which authors have demonstrated the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions. An excellent work is valuable for malaria vector control program.
Details on Page 497
Objective:To determine the efficacy of WHO impregnated paper and CDC coated bottle based on number of storage days and number of times of consecutive use, in the assessment of insecticide vector susceptibility tests in laboratory and field conditions.
Efficacy, Coated bottle, Impregnated paper, Insecticide, Laboratory conditions, Field conditions
1. Introduction
Malaria is a severe public health problem, causing an estimated 225 million disease cases and 781 000 deaths per year[1]. Most victims are children under five years old living in sub-Saharan Africa[1]. Malaria is transmitted byAnophelesmosquitoes, and because there is currently no vaccine available, vector control is one of the mostimportant means of malaria prevention. This vector control is generally done with insecticides. In Benin, as across Africa, malaria control relies heavily on vector control through the use of insecticide-treated nets and indoor residual spraying.
The shelf-life and re-use of pre-prepared bottles are still not well documented or studied in laboratory conditions[2]. Studies carried out in the Peruvian laboratory showed that pyrethroid-treated bottles could be used several times and stored for long periods under ambient conditions, but this finding will be less applicable to the highly volatile organophosphates[3]. However, these authors did not clearly mention in their published paper, the shelf-life and re-use of pre-prepared bottles with pyrethroids. It is useful to check this report or finding from these authors. Similar to CDC bottles, WHO impregnated papers are regularly used in the assessment of insecticide susceptibility tests in mosquito vectors natural populations. WHO reference centre at the Vector Control Research Unit or on behalf of WHO by the University Sains Malaysia, which is based in Penang, Malaysia does not generally indicate on the packaging of these papers, the shelf-life and re-use of these WHO impregnated papers.
The aim of this study was to determine the efficacy of a Wheaton coated bottle with permethrin and of a Wheaton coated bottle with deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions, in order to define some strategies which would help to reduce or economize the amount of insecticide useful in the assessment of insecticide vectors susceptibility tests in laboratory and field conditions.
2. Materials and methods
2.1. Study area
The study was carried out in south and north of Benin. In southern Benin, it was carried out in in Seme-Kpodji district located in Oueme department and in Suru-lere locality in Cotonou district located in Littoral department. In northern Benin, it was carried out precisely in Parakou district located in Borgou department (Figure 1). Parakou district is far from Cotonou about 400 km. Study sites were chosen based on the economic activities of populations, their usual protection practices against mosquito bites, and peasant practices to control farming pests. Seme-Kpodji is in Oueme region characterized by a subequatorial type climate with four seasons, two rainy seasons (March-July and September-November) and two dry seasons (December-March and August-September).
The temperature ranges from 25 to 30 °C with the annual mean rainfall between 900 and 1 500 mm. Suru-lere is in Cotonou district located in Littoral region characterized by a tropical coastal Guinean climate with two rainy seasons (April-July and September-November). The mean annual rainfall is over 1 500 mm. Parakou is in Borgou region characterized by a Sudanian climate with only one rainy season per year (May to October) and one dry season (November-April). The temperature ranged from 22 to 33 °C with the annual mean rainfall which is 1 300 mm.

Figure 1. Map of the study area.
2.2. Mosquito sampling
Anopheles gambiae(An. gambiae)s.l. mosquitoes were collected in April 2013 during the first rainy season in Seme-kpodji and Cotonou districts located in Southern Benin.An. gambiae s.l. mosquitoes were also collected in May 2013 at the beginning of the rainy season in Parakou district located in Northern Benin. Larvae and pupae were collected using the dipping on breeding sites and then kept in separated labeled bottles related to each locality. The samples from Seme-Kpodji and Cotonou districts were reared up to adult emergence at the CREC insectary whereas the samples from Parakou district were reared up to adult emergence in a hotel located in Parakou district.
2.3. Diagnostic doses of insecticides
Bendiocard was used to impregnating WHO paper. It isthe most commonly used insecticide for indoor residual spraying in Northern Benin. Insecticide diagnostic dose applied were based on WHO recommendations[4]. The discriminating dosage selected was twice the experimentally derived 100% lethal concentration (LC100) value of a reference susceptible strain. This dose was applied onAn. gambiaeKisumu, reference susceptible strain before being applied on field populations (An. gambiaeParakou). A diagnostic dose of 0.1% was applied onAn. gambiae, for 60 min exposure period.
Regarding CDC bioassays, application of diagnostic doses were those recommended by CDC[5]. These doses were applied onAn. gambiaeKisumu, reference susceptible strain before being applied on field populations (An. gambiaeSeme-Kpodji andAn. gambiaeSuru-lere). ForAn. gambiae s.l., the diagnostic dose 21.5 µg of permethrin per bottle and 12.5 µg of deltamethrin per bottle were applied for 120 min exposure period and for a diagnostic time of 30 min. The choices of permethrin and deltamethrin were based on widespread use of both insecticides on OlysetNets and permaNets 2.0 respectively. The NMCP distributed OlysetNets across the country for free in July 2011, while the free distribution of permaNets was carried out in Oueme department only, before July 2011.
2.4. Preparation of stock solutions
The solutions which were used for CDC bottles coating were a mix of stock solution and acetone. For example, to prepare the stock solution of deltamethrin 12.5 µg per bottle, we weighed 12.5 mg of deltamethrin which was dissolved in 1 L of acetone. The solution was stirred up until complete dissolution of the powder and the whole mixture homogenized. Solutions were stocked in no sensitized light bottles and put at refrigerator until their use.
2.5. Wheaton bottles coating
The bottles coating was done by following the protocol described by CDC[5,6]. After Wheaton bottles and caps had cleanly been washed and completely well sun-dried, each bottle and its cap were labeled (by the same number, the same insecticide name, and the same name of strain which has to be tested). Once the stock solutions prepared, they were stirred lightly to be homogenized before their use. About 1 mL of acetone was added to the control bottle and the cap was put back on tightly; and then 1 mL of the stock solution of permethrin or deltamethrin was added to the test bottle and the cap was also put back on tightly to avoid acetone evaporation. The contents inside the bottle was swirled so that the bottom was coated, then the bottle was inverted and swirled to coat the inside of the cap. The bottle was placed on its side for a moment to let the contents pool. Then the bottle was gently rotated while rocking so that all the sides around were coated. The cap was removed and bottle continued to be rolled on its side until all visible signs of the liquid were gone from inside and the bottle was dry. Finally, the control and test bottles were left on their sides on the lab bench of manipulation in the laboratory, where they were protected from light until they were completely dried. Each cap was put in front of corresponding bottle and the inside of these caps facing the sky.
2.6. Performing the efficacy tests
CDC bioassays were performed with a 250 mL Wheaton coated bottle (new or clear bottle) with permethrin at diagnostic dose 21.5 µg per bottle. One coated bottle with acetone only served as control. This dose was checked on theAn. gambiaeKisumu susceptible reference strain before being applied to field populations (An. gambiaeSuru-lere) reared from the larval and pupal collections to adults in the CREC insectary.
These bioassays were performed like this: an aspirator was used to introduce 15 to 20 unfed female mosquitoes from Suru-lere and Sekandji aged 2-5 days old into a 250 mL Wheaton coated bottle with permethrin or deltamethrin. The number of dead or alive mosquitoes was monitored at different time intervals (15, 30, 35, 40, 45, 60, 75, 90, 105, 120 min). The percent mortality at susceptibility threshold (30 min) was determined. These CDC bioassays were performed during several consecutive days under ambient temperature and relative humidity conditions in laboratory (which were each time recorded during each test at laboratory) until the coated bottle lost its efficacy. Bioassays were stopped as soon as the percent mortality recorded in the test bottle was inferior to 100% with bothAnophelespopulations collected in the field,An. gambiaeSuru-lere andAn. gambiaeSekandji populations. Both field and reference populations susceptible to permethrin since the first CDC test were used in tests repetition. After each test, a line was marked on the label of the test bottle. This bottle was well kept aside under good temperature and relative humidity conditions in laboratory.
As for the permethrin, the same procedure was followed in bioassays repetition with deltamethrin and with the same CDC method. WHO susceptibility tests were performed with a new impregnated paper with bendiocarb. Two tubes containing non-impregnated papers served as controls.
These bioassays were performed like this: an aspirator was used to introduce 20 to 25 unfed female mosquitoes aged 2-5 days old, reared from the larval and pupal collections to adults in a Parakou hotel, into a WHO test tube that contained an impregnated paper with bendiocarb. The number of dead mosquitoes was monitored at different time intervals (5, 10, 20, 30, 40, 50, 60 min). The percent mortality was determined 24 h after the performing of WHO susceptibility test. These bioassays were repeated during several consecutive days with the same impregnated paper with bendiocarb in a Parakou hotel in the evening under ambient field conditions. These conditions (temperature and relative humidity) were recorded in the field during each susceptibility test until the impregnated paper lost its efficacy. At the end of the WHO bioassays, mosquitoes were transferred to the holding tubes and were each time provided with cotton wool moistened with a 10% honey solution. Tubes containing these mosquitoes were put on a table whose legs were immersed in water in the hotel for 24 h mortality recording. WHO susceptibility tests were first assessed withAn. gambiaeKisumu, susceptible reference strain carried from CREC insectary to Parakou hotel, and which served as control following the same procedure as the one used withAn. gambiaeParakou.
2.7. Statistical analysis
The resistance status of mosquito samples from Parakou was determined according to the latest WHO criteria as follows[4]: Mortality rates between 98%-100% indicate full susceptibility; Mortality rates between 90%-97% require further investigation; Mortality rates <90%, the population is considered resistant to the tested insecticides. The resistance status of mosquito samples from Sekandji and Suru-lere was determined according to the CDC criteria[5,6]. The susceptibility thresholds at the diagnostic time of 30 min for pyrethroids are:
Mortality rate=100%: the population is fully susceptible.
Mortality rate<100%: the population is considered resistant to the tested insecticides.
Abbott’s formula was not used in this study for the correction of mortality rates in either the test-tubes or test-bottles because the mortality rate in all controls was always less than 5%[7].
Analysis using Fisher’s exact test for equality of two proportions was performed on the data sets gathered from the localities surveyed to compare for each of three tested insecticides, the mortality rate ofAn. gambiaepopulations obtained during the first susceptibility test to the one obtained during the repeated tests. Data are presented with 95% confidence limits.
3. Results
3.1. Efficacy of a Wheaton coated bottle with deltamethrin in laboratory conditions
The analysis of Table 1 shows thatAn. gambiaeKisumu population, after four susceptibility tests during four consecutive days in insecticide testing laboratory at CREC, remained susceptible to deltamethrin with the mortality rate of 100%. After 30 min in CDC coated bottle with deltamethrin, which represents susceptibility threshold time or diagnostic time clearly defined by CDC protocol,An. gambiaeKisumu mosquitoes were knocked-down. RegardingAn. gambiaeSekandji population, after three susceptibility tests during three consecutive days in laboratory, these mosquitoes remained susceptible to deltamethrin with the mortality rate of 100%. The mortality rate recorded during the fourth susceptibility test was 95.23% (P>0.9999). There were no differences between dates. The temperature mean recorded during these susceptibility tests was 25 °C. The relative humidity mean was 75% (Table 1).

Table 1 Efficacy of a Wheaton coated bottle with deltamethrin 12.5 µg/bottle in laboratory conditions.
3.2. Efficacy of a Wheaton coated bottle with permethrin in laboratory conditions
The analysis of Table 2 shows thatAn. gambiaeKisumu population, after four susceptibility tests during four consecutive days in insecticide testing laboratory at CREC, remained susceptible to permethrin with the mortality rate of 100%. After 30 min in CDC coated bottle with permethrin, which represents susceptibility threshold time or diagnostic time clearly defined by CDC protocol,An. gambiaeKisumu mosquitoes were knocked-down. RegardingAn. gambiaeSuru-lere population, after one susceptibility test during one day in laboratory, these mosquitoes remained susceptible to permethrin with the mortality rate of 100%. The mortality rates recorded during the second, third and fourth susceptibility tests were 83.33% (P=0.478), 73.33% (P=0.113) and 78.94% (P=0.268) respectively. There were no differences between dates. The temperature mean recorded during these susceptibility tests was 25 °C. The relative humidity mean was 75% (Table 2).

Table 2 Efficacy of a Wheaton coated bottle with permethrin 21.5 µg/bottle in laboratory conditions.
3.3. Efficacy of aWHOimpregnated paper with bendiocarb in field conditions
The analysis of Table 3 shows thatAn. gambiaeKisumu andAn. gambiaeParakou populations, after four susceptibility tests during four consecutive days in the field, remained susceptible to bendiocarb with the mortality rate of 100%. There were no differences between dates. The 24 h mortality recording required by WHO protocol shows that all these mosquitoes were dead, and none of them could fly. The temperature mean recorded during these susceptibility tests was 27 °C. The relative humidity mean was 90% (Table 3).
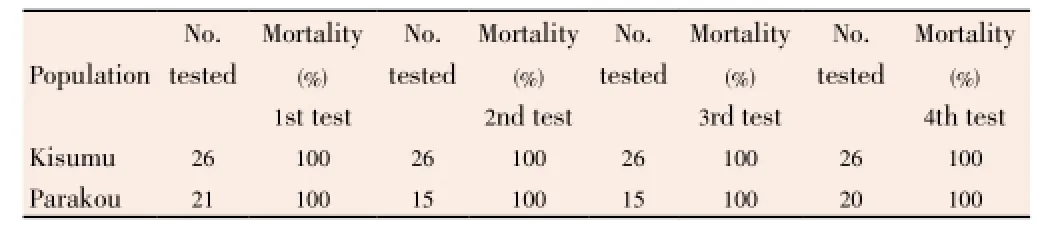
Table 3 Efficacy of a WHO impregnated paper with bendiocarb 0.1%in field conditions.
4. Discussion
Study of the efficacy of a Wheaton coated bottle with insecticide in laboratory conditions and the study of the efficacy of a WHO impregnated paper with insecticide in field conditions were very important because they allow to reduce or economize the amount of insecticide necessary in the assessment of the insecticide vectors susceptibility tests.
In the current study, the temperature mean recorded during CDC bioassays in insecticide testing laboratory at CREC was 25 °C with a 75% relative humidity mean. The temperature mean recorded during WHO bioassays in the field, was 27 °C with a 90% relative humidity mean. Temperatures’ recording during susceptibility tests was very important because ambient temperature can influence the toxicity of insecticides[4]. Similarly, the relative humidity has been shown to affect the survival of mosquitoes during the holding period. It is therefore recommended, as previously, that temperature and humidity are controlled during the test and holding periods. If possible, tests should be carried out at (25±2) °C and (80±10)% relative humidity[4]. The temperature should never exceed 30 °C because a very high temperature can conduct to an over-estimate of vectors resistance level to insecticides. In this study, temperature and relative humidity recorded during the susceptibility tests were within the intervals required by WHO[4].
An. gambiaeKisumu populations (control) after four CDC susceptibility tests during four consecutive days in insecticide testing laboratory at CREC remained susceptible to permethrin and deltamethrin. These results show thatAn. gambiaeKisumu populations arrived at the CREC insectary in 1999 were well reared up and stored in good conditions in Benin. A Wheaton treated bottle with 12.5 µg a.i. deltamethrin per bottle and 21.5 µg a.i. permethrin per bottle could be used at least three times during four consecutive days in laboratory conditions. After the fourth day, bottles have to be washed and re-coated before their re-use.An. gambiaeParakou populations, after four susceptibility tests during four consecutive days in the field, remained susceptible to bendiocarb. A WHO impregnated paper with bendiocarb could be used four times during four consecutive days in field conditions. On the fifth use, the paper still maintained its efficacy. In field conditions, the studies of Pereaet al.showed that treated bottles with 10 µg a.i. deltamethrin per bottle could be stored for at least 14 d and re-used on three occasions if these bottles had been capped and stored in the dark at ambient temperatures in the field[8]. These authors showed that after three uses, they appear to lose effect. That was presumably due to the redistribution of insecticide caused by contact with mosquitoes, aspirators and moisture from the air or from mouth aspiration. These authors also showed that wherever bottles are to be pre-prepared and stored, similar tests should be used to define their effective shelflife. Regarding impregnated papers, WHO recommended that none insecticide-impregnated paper should be used no more than 6 times[4]. The efficacy of a Wheaton coated bottle with insecticide or a WHO impregnated paper with insecticide also depend on insecticide vectors susceptibility; the reason why it was important was that it first knew vector (local vector) susceptibility to insecticide before the repetition of the susceptibility tests with the same treated bottle or the same impregnated paper.
Treated bottle or impregnated paper with insecticide storage also has an important role in the efficacy study. In this study, after the assessment of each susceptibility test in laboratory, bottles were capped and stored in a cardboard and put in a safety place in order to prevent them from breakages before the next test. Regarding WHO impregnated papers, after the assessment of each susceptibility test in the field, they were put in theirpackaging and stored in a cardboard and put in a safety place in the hotel.
In the current study, we have not investigated the costs of WHO and CDC kits useful in the assessment of insecticide vectors susceptibility tests. However, CDC kit may be cheaper than WHO kit[8].
In conclusion, study of the efficacy of a Wheaton coated bottle with insecticide in laboratory conditions and a WHO impregnated paper with insecticide in field conditions was useful in the assessment of insecticide vectors susceptibility tests. The ambient temperature and relative humidity recording in laboratory and in field during susceptibility tests were also neccessary.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Acknowledgements
The authors would like to thank the Ministère de l’Enseignement Supérieur et de la Recherche Scientifique (MESRS), Benin for supporting the doctoral training of Nazaire. We would like to thank Dr. William G. Brogdon from CDC Atlanta who supplied us the reagents used for CDC bioassays. The authors would also like to thank Frédéric OKE-AGBO for statistical analysis, Damien Todjinou for providing technical assistance. This research was funded by the Ministère de l’Enseignement Supérieur et de la Recherche Scientifique (MESRS), Benin and the President’s Malaria Initiative of the U.S. Government through USAID.
Comments
Background
This study would help to reduce or economize the amount of insecticide useful in the assessment of insecticide vectors susceptibility tests in laboratory and field conditions.
Research frontiers
The present research work depicts a good entomological study and valuable for the resistance monitoring of vectors.
Related reports
In field conditions, some authors showed that treated bottles and used three times appear to lose effect.
Innovations and breakthroughs
A WHO impregnated paper with bendiocarb could be used four times during four consecutive days in field conditions. On the fifth use, the paper still maintained its efficacy. The efficacy of a Wheaton coated bottle with insecticide or a WHO impregnated paper with insecticide also depend on insecticide vectors susceptibility.
Applications
It can be applied in entomological study for the assessment of insecticide vectors susceptibility tests in laboratory and field conditions in malaria-endemic countries.
Peer review
This is a valuable research work in which authors have demonstrated the efficacy of a Wheaton coated bottle with permethrin and deltamethrin in laboratory conditions and a WHO impregnated paper with bendiocarb in field conditions. An excellent work is valuable for malaria vector control program.
[1] World Health Organization. World malaria report. Geneva: World Health Organization; 2010. [Online] Available from: http:// www.who.int/malaria/world_malaria_report_2010/en/. [Accessed on 25th November, 2013]
[2] Aïzoun N, Ossè R, Azondekon R, Alia R, Oussou O, Gnanguenon V, et al. Comparison of the standard WHO susceptibility tests and the CDC bottle bioassay for the determination of insecticide susceptibility in malaria vectors and their correlation with biochemical and molecular biology assays in Benin, West Africa. Parasit Vectors 2013; 6: 147.
[3] Devine GJ, Ogusuku E. Adaptability is key when monitoring insecticide resistance. Bull World Health Organ 2009; 87: 887.
[4] World Health Organization. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. Geneva: World Health Organization; 2013. [Online] Available from: http://www.who.int/malaria/publications/atoz/9789241505154/en/. [Accessed on 24th December, 2013]
[5] Brogdon W, Chan A. Guidelines for evaluating insecticide resistance in vectors using the CDC bottle bioassay/methods in Anopheles research. 2nd ed. CDC technical report 343. Atlanta: CDC Atlanta USA; 2010.
[6] Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc 1998; 14(2): 159-164.
[7] Abbott WS. A method of computing the effectiveness of an insecticide.1925. J Am Mosq Control Assoc 1987; 3(2): 302-303.
[8] Zamora Perea E, Balta León R, Palomino Salcedo M, Brogdon WG, Devine GJ. Adaptation and evaluation of the bottle assay for monitoring insecticide resistance in disease vector mosquitoes in the Peruvian Amazon. Malar J 2009; 8: 208. doi: 10.1186/1475-2875-8-208.
10.12980/APJTB.4.2014C1111
*Corresponding author: Nazaire Aïzoun, Centre de Recherche Entomologique de Cotonou (CREC), 06 BP 2604, Cotonou, Bénin.
Tel: (229) 95317939
E-mail: aizoun.nazaire@yahoo.fr
Foundation Project: Funded by the Ministére de l’Enseignement Supérieur et de la Recherche Scientifique (MESRS), Benin and the President’s Malaria Initiative of the U.S. Government through USAID.
Article history:
Received 15 Mar 2014
Received in revised form 3 Apr, 2nd revised form 9 Apr, 3rd revised form 20 Apr 2014
Accepted 12 May 2014
Available online 28 Jun 2014
Methods:Larvae and pupae of Anopheles gambiae s.l. mosquitoes were collected from the breeding sites in Seme-Kpodji and Cotonou districts in Southern Benin in April 2013 during the first rainy season. Anopheles gambiae s.l. mosquitoes were also collected from the breeding sites in Parakou district in Northern Benin in May 2013 at the beginning of the rainy season. Susceptibility tests were done using impregnated paper with bendiocarb (0.1%) following WHO protocol and stock solutions of permethrin (21.5 µg per bottle) and deltamethrin (12.5 µg per bottle) following CDC protocol on unfed female mosquitoes aged 2-5 days old. These bioassays were repeated a certain number of times. The temperature and relative humidity were monitored and recorded during the susceptibility tests.
Results:This study showed that a WHO impregnated paper with bendiocarb could be used four times during four consecutive days in field conditions. Regarding a Wheaton coated bottle with permethrin or deltamethrin, they could be used at least three times during four consecutive days in laboratory conditions.
Conclusions:The day storage and the number of times that a WHO impregnated paper and a CDC coated bottle maintained their efficacy are useful in the assessment of insecticide vectors susceptibility tests.
 Asian Pacific Journal of Tropical Biomedicine2014年6期
Asian Pacific Journal of Tropical Biomedicine2014年6期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- A retrospective evaluation of the quality of malaria case management at twelve health facilities in four districts in Zambia
- Pharmacognostical study and establishment of quality parameters of aerial parts of Costus speciosus-a well known tropical folklore medicine
- Hepatocurative potential of Vitex doniana root bark, stem bark and leaves extracts against CCl4-induced liver damage in rats
- In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit
- Antimicrobial activity of some essential oils against oral multidrugresistant Enterococcus faecalis in both planktonic and biofilm state
- Phytochemical and biological studies of Butia capitata Becc. leaves cultivated in Egypt
