PRP and Metaplasia in repaired tendon
Plasric & Reconstructive Surgery, Iran University of Medical Sciences, Tehran, Iran
PRP and Metaplasia in repaired tendon
Kamal Seyed-Forootan, Hamid Karimi, Ahmad-Reza Dayani
Plasric & Reconstructive Surgery, Iran University of Medical Sciences, Tehran, Iran
Objective: To evaluate effects of PRP injection in strengthening of repaired tendon. Methods: This study was conducted in animal lab of our hospital on 20 rats. The animals were divided into two groups randomly and distal third of left Achillis tendons were cut and then repaired with Vicryl 2/0. The first group was control group and in the second group 0.5 cc of PRP was injected into the repair site. After 4 weeks all of the rats were executed and 70% of tendons were sent randomly for tensilometry and the force that required to rupture the tendons were measured. In next stage the tendons were sent for pathological exam. Results: The force that was needed to rupture the tendon were not significantly different in the two groups. Neovascularization were more prevalent in PRP group but not statistically significant. There were two cases of Cartilage Metaplasia in PRP group. Conclusion: It seems that using PRP has no effect on strengthening the tendons repair and may have some adverse effects. It usage needs further studies to evaluate their probable adverse side effects.
ARTICLE INFO
Article history:
Received 9 November 2014
Received in revised form 15 December 2014
Accepted 18 December 2014
Available online 20 January 2015
Trauma
Tendon
Achillis
Repair
PRP
Tensilometry
1. Introduction
Achillis is the largest tendon of the body and has an important role in stabilityof body, posture and walking. trauma to this tendon is very common especially in Atelets. The treatment modalities for tendon rupture are diverse.
These treatments are not complete and have side effects and sometime is with pain after the treatment[1]. One of the new modalities is PRP injection in the repair site.
It is shown that PRP can help in healing process of soft and hard tissues and has been used in Orthopedics, Dentistry, Dermatology, Plastic and Maxillofacial surgeries[2]. Some of the studies shown the mitogenic effects of PRP and have emphecised on the role of the growth factors in PRP[3].
Adhesions is one the problems after tendon repair and needs early movement of tendon ,although early movement may induce rupture of the tendon[4,5]. To overcome these effects some authors suggest using growth factors to strenghten the repair site and early start of tendon movement. we know that PRP has TGF and VEGF and can induce chemotaxis, fibroblast proliferation, collagen synthesis, cell proliferation and increase of angiogenesis.
PRP is an autologus, non expensive and simple tissue that has regeneration power, it has no allergic or infectious side effects and it is shown that PRP would remain for one week in the repair site, so it seemsthat one dose of injection would be enough for tendon repair[6-15].
2. Materials and methods
The study was performed on 20 Vaster albinoc male rats, weighting 200-250 g and age about 2 months. The rats were kept in separate cages with 12 h day and night cycle and tempreture of 24-28 degree of centigrade and enough food and water. The animals were kept under Helsinky protocol. All of the surgeries were done by the same surgeon, Rats were anesthetized with Ketamin 15 mg/kg and Xilocaine 5 mg/kg. Ceftriaxone 60 mg/kg IM were injected into the rats before surgery. 2.5 cc of Blood were taken from the rats andcentrifuged and the PRP was isolated with pipette .
Incision were made over distal third of Achillis tendon and tendons disected and cut in distal third and then repaired with Vicryl 4/0 (Figure 1). In first group no other treatment was done but in second group 0.5 cc of PRP were injected in the repair site then skin closed with nylon 4/0. Splint applied over the legs and rats were kept in the cages and were free to move. The rats were examined every other day by a physician. None of the rats died during the study. No infection were seen during the study.
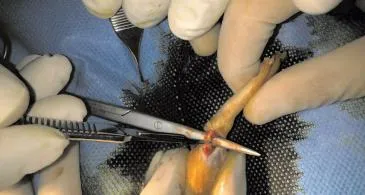
Figure 1. Cuting of the Aschillis tendon.
After 28 days all of the rats executed with CO2gas without any pain. And the tendons were removed with calcaneous bone. Seben tendons in each group were selected randomly and sent for tensilometry (Figure 2). The force that needed to rupture the tendons were measured in newton.
Five mm by 5 mm of the tendons in the repair site were sent for pathologic exam. The pathologist were blinded about the two groups. The samples were colored with hematoxilin and eosin and studied for the number of fibroblasts, collagenes and their orientation and vascularization. Photographies were taken from the samples.
The results were expressed as mean±Standard deviation and analyzed with SPSS 16 software.P<0.05 were considered significant. For analysing ANOVAtest, Mann-Whitney, Wilcoxon, Tukey and Dunnet test were used.

Figure 2. Tendon samples for tensilometry.
3. Results
All of the rats were alive after 28 days. The mean force that was needed for tendon rupture were 47 newtons in control group and 57 newtons in PRP group (P=0.193). In the control group in 2 tendons, rupture happened with force of 40 newtons. But none of the tendon in PRP group rutured with forces less than 40 newtons. The maximum force in control group was 70 newtons and in PRP group 82 newtons. In 28% of control group rupture happened with forces less than 40 newtons. And in PRP group, 14% ruptured with forces morethan 80 newtons (Table 1 & 2).

Table 1 Comparison of forces that need to rupture the tendons after 4 weeks (P=0.193).

Table 2.Comparison of mean of forces that need to rupture the tendons between the two groups (P=0.193).

Table 3
On average the force that needed for rupture of tendons in PRP group were 20 % more than the control group (Figure 3). The samples were examined for number of acute and chronic phase inflammatory cells, fibroblast, fibrosis, collagene fibers orientation,vascularization and metaplasia (Fiugre 4 & 5). Acute phase infalamatory cells were not found in the samples.

Figure 3. Comparison of the tensile strenght between the two groups.

Figure 4. Neo-vascularization.
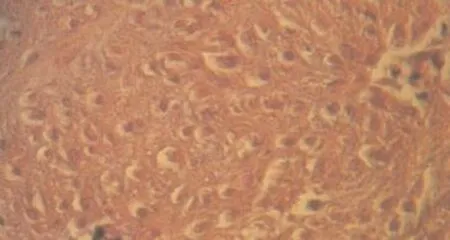
Figure 5. Collagen fibrils and orientation.
Fibroblast orientation were not diferent in both groups. Number of fibroblasts were shown in table 3. The difference in the two groups were not significant. In PRP group two cases of Metaplasia were seen, cartilage Metaplsia was seen in two samples (Figure 6 & 7).

Figure 6. Cartilage metaplasia.

Figure 7. Cartilage metaplasia after using PRP in tendon.
4. Discussion
Musculoskeletal injuries are the most common cause of severe long-term pain and physical disability, and affect hundreds of millions of people around the world. Therapeutic approaches to tendon healing do not always result in a satisfactory anatomical and functional repair, and healed tendon is often characterized by functional impairment and high risk of reinjury. Recently, mesenchymal stem cells (MSCs) and platelet rich plasma (PRP) have been proposed as novel therapeutic treatments to improve the tendon repair process. MSCs are multipotent, easy to culture and being originated from adult donors do not pose ethical issues. To date, autologous MSCs have been investigated mainly in the treatment of large bone defects, cardiovascular diseases, osteogenesis imperfecta and orthopaedic injuries both in human and veterinary medicine[4].
One of the most popular methods used to biologically enhance healing in the fields of hand surgery, orthopaedic surgery and sports medicine includes the use of autologous blood products, namely, platelet rich plasma (PRP). PRP is an autologous concentration of human platelets to supraphysiologic levels. At baseline levels platelets function as a natural reservoir for growth factors including plateletderived growth factor (PDGF), epidermal growth factor (EGF, transforming growth factor-beta 1 (TGF-β1), vascular endothelial growth factor(VEGF), basic fibroblast growth factor (FGF), hepatocyte growth factor (HGF), and insulin-like growth factor (IGF-I). Platelet-rich plasma (PRP) is derivedfrom centrifuging whole blood, has a platelet concentration higher than that of the whole blood, is a part of the cellular component of plasma that settles after centrifugation[3]. and now a days is commonly used in orthopaedic practice to augment healing in sports-related injuries of skeletal muscle, tendons, and ligaments. Despite its pervasive use, the clinical efficacy of PRP therapy and varying mechanisms of action have yet to be established. Basic science research has revealed that PRP exerts is effects through many downstream events secondary to release of growth factors and other bioactive factors from its alpha granules. These effects may vary depending on the location of injury and the concentration of important growth factors involved in various soft tissue healing responses. Current findings in PRP basic science research, which have shed light on its proposed mechanisms of action, have opened doors for future areas of PRP research. We know that specific growth factors may play a crucial role in the healing process. There is abundant enthusiasm in the application of concentrated platelets, which release a supra-maximal quantity of these growth factors to stimulate recovery in injuries.
There is increasing interest in the sports medicine and athletic community about providing endogenous growth factors directly to the injury site using autologous blood products such as PRP, to potentially facilitate healing and earlier and faster return to sport after musculoskeletal injury[3].
Platelet rich plasma (PRP) , the autologous plasma fraction with a platelet-rich cellular component, Due to its availability and low cost, has become an increasingly popular clinical tool as an alternative source of growth factors for various applications, for example tendon regeneration but with limited success in clinical trials[5]. Plateletrich plasma (PRP) therapy aims to improve the process of tissue repair through local delivery of autologous bioactive agents to influence critical physiological mechanisms such as inflammation angiogenesis, or extracellular matrix synthesis. These biological properties have prompted the therapeutic administration of PRP in reconstructive and sports medicine. Given its biocompatibility and healing properties, percutaneous injections of PRP are also used in athletes to treat tendon and muscle injuries[30] and also for the treatment of ligament, cartilage injuries and early osteoarthritis[38], lateral epicondylitis, ligament and muscle strains, and tears of the rotator cuff anterior cruciate ligament, and Achilles tendon[16]. Platelet-rich plasma can be applied at the site of injury either during surgery or through an injection performed in the physician’s office. And many investigators are exploring the ways in which this therapy can be used in the clinical setting. However, there is little published clinical evidence that proves its efficacy in treating the multitude of injuries/disorders that are thought to benefit from PRP[16]. the potential risks involved with PRP are fortunately very low. However, benefits remain unproven to date[3]. PRP use in tendon and ligament injuries has several potential advantages, including faster recovery and, possibly, a reduction in recurrence, with no adverse reactions described[17]. Some results indicate that PRP exerts anti-inflammatory effects on injured tendons through HGF. This study provides basic scientific evidence to support the use of PRP to treat injured tendons because PRP can reduce inflammation and thereby reduce the associated pain caused by high levels of PGE2[18] and reduction in pain may facitilate earlymovement of tendon and limb.
In 2010. Zhanget alreported that PRP treatment of injured tendons is “safe” as it promotes TSC differentiation into tenocytes rather than nontenocytes, which would adverse effects on the structure and function of healing tendons by formation of nontendinous tissues. Moreover, they suggest that PRP treatment can enhance tenocytes to proliferate quickly and produce abundant collagen to repair injured tendons[19].
PRP secretes many growth factors, including transforming growth factor beta1 (TGF-beta1), platelet derived growth factor, vascular endothelial growth factor, insulinlike growth factor 1, and so on, which can induce cell proliferation, chemotaxis, and collagen synthesis for wound healing. it is shown that PRP can facilitate rabbit’s tendons healing and significantly improve the healing quality[20]. PRP is also used in conjunction with other product such as: TCA[21], MSCs [22], ASCs[23], fibrin matrix[24], TSC tendon stem cells[25], Leukocyte- and platelet-rich plasma[26], The“Cascade” membrane a thin layer of autologous fibrine that is very rich in platelets and is obtained by high speed centrifugation of a small quantity of PRP[27].
The repair process of tendon injuries, is slow and the quality of the repair tissue is often inferior to the original tendon tissue, which frequently leads to re-injury. The relatively poor vascularization of tendons is one of the reasons. PRP, rich in growth factors, enhance the vascularization.The vascular endothelial growth factor, a powerful stimulator of angiogenesis, is abundantly present in PRP suggesting that enhancement of neovascularization might be one of the mechanisms. Boschet alin 2011 shown that PRP induced significantly more neovascularization than the placebo treatment until at least 23 weeks after treatment, as detected by both Doppler ultrasonography and Factor VIII staining long-lasting effect[28] and it has Lyras et al also stated that The poor vascularity of tendons is a major factor in their limited healing capacity. Their study shown significantly more angiogenesis in the PRP group during the first two weeks of the healing process, i.e inflammatory and proliferative phase .
The number of the newly formed vessels in the PRP group were significantly reduced at 4 weeks compared to the controls (P<0.0001) meaning that the healing process was shortened. And concluded that PRP may accelerate the healing process and promote scar tissue of better histological quality[29]. Histologically it is seen that healing process in the tendons of PRP group is faster. In the first 2weeks of healing, IGF-1 was found intracellularly in various type cells, whereas in the last 2 weeks of healing IGF-1 was detected mainly in tenocytes. Both cytoplasmic and nuclear expressions were present, whereas the larger amounts of immunoexpression were localized in both epitenon and endotenon. And it was concluded that PRP may improve tendon defect healing by overexpression of IGF-1[30].
In early phases of tendon healing processes, circulationderived cells temporarily exist in the wounded area to initiate the healing process and decrease in number with time. In a study Kajikawa et al said that a delay of time-dependent decrease in circulation-derived cells could improve the healing of tendons. They injected PRP into the wounded area of the patellar tendon, and compared the effects on activation of circulation-derived cells and enhancement of tendon healing and concluded that locally injected PRP is useful as an activator of circulation-derived cells for enhancement of the initial tendon healing process[31]. The repair of subcutaneous tendon ruptures can be stimulated by a single application of one of several growth factors [e.g. platelet-derived growth factor (PDGF), transforming growth factor (TGF)-beta, insulin-like growth factor (IGF)-1, vascular endothelial growth factor (VEGF), bone morphogenetic proteins (BMPs) like growth differentiation factor (GDF)-5, -6, -7]. The response to these measures is dependent on the mechanical microenvironment. So far, almost all research has been limited to rodent models, mostly using the rat Achilles tendon[32].
PRP increases metabolic activity and seems to advance maturation of repair tissue over nontreated experimentally induced tendon lesions, which suggests that PRP might be beneficial in the treatment of clinical tendon injuries[33]. Also Mechanical stimulation improves the repair of ruptured tendons. Injection of PRP can also improve repair in several animal models. In a rat Achilles tendon transection model, one postoperative injection resulted in increased strength after 4 weeks. Considering the short half-lives of factors released by platelets, this very late effect calls for an explanation that Platelets influence only the early phases of regeneration, but this allows mechanical stimulation to start driving neo-tendon development at an earlier time point[34]. Many commercial preparation systems for PRP therapy are available, but the optimal preparation remains unknown. Increasing numbers of clinical studies evaluating PRP have been reported and have provided both positive and negative evidence for its effectiveness. Well-designed, controlled studies are still lacking, but PRP may have a benefit for patients with tendinopathy that is refractory to other nonsurgical treatments. Its use in tendon repair is currently not supported
Randomized, controlled studies with documentation of platelet, white blood cell, and growth factor concentration in the PRP preparation are necessary for future comparative research[35]. However, whatever is the machanism of action of PRP, the result has to be a stenghthen and strong tendon that would not rupture again. There are reports that an injection of PRP in sectioned rat Achilles tendon influences the early phase of tendon healing and results in an ultimately stronger mechanical resistance[34,36,37]. And in contrast other studies showed that a single injection of PRP appears not useful for Achilles rat tendon tear[38,39]
In this study we examined the result of using PRP and measured the force that are needed to re-rupture the newly repaired tendon. Our results showed that the tensile strenght of tendons in PRP group are more but not significantly different. Also we examined the samples histologically to find any sign of remained or delayed healing process in the tendons. The results revealed that the healing process are complete after 4 weeks in both groups and there is not any delay in its process. The collagen fibers and their orientation are the same. The orientation of collagen fibers in the PRP group was better organized but not different significantly.
And interestingly the vascularization in the two groups were not different significantly. Therefore our result does not support using PRP as an adjuvant in treatment of tendon ruptures. PRP has not strenghtened the tendon repair site and did not improve the mechanical properties of repaired tendon. More over accidental finding of two cases of metaplasia is a warning alert sign. Existance of Metaplasia in treatment site is a new finding (complication?)and varant further comprehensive studies in this kind of therapy .
PRP does not improve tendon rupture healing. It seems that all aspects of using PRP are not completely underestood and further studies are still necessary to find its role in healing procees. Cartilage metaplasia, as far as we know, hasnot been reported yet in PRP treatment and may have some adverse effects on the results of tendon repair.
Conflict of interest statement
We declare that we have no conflict of interest.
Refereneces
[1] Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J 2012; 32: 150-163.
[2] Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med 2008; 1(3-4): 165-174.
[3] Paoloni J, De Vos RJ, Hamilton B, Murrell GA, Orchard J. Platelet-rich plasma treatment for ligament and tendon injuries. Clin J Sport Med 2011; 21(1): 37-45.
[4] Ricco S, Renzi S, Del Bue M, Conti V, Merli E, Ramoni R. Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol 2013; 26(1 Suppl): 61-68.
[5] Wang X, Qiu Y, Triffitt J, Carr A, Xia Z, Sabokbar A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J Orthop Res 2012; 30(6): 982-990.
[6] Mishra A, Woodall J Jr, Vieira A. Treatment of tendon and muscle using Platelet-rich plasma. Clin Sports Med 2009; 28(1): 113-125.
[7] Kon E, Filardo G, Delcogliano M. Platelet-rich plasma: New clinical application: A pilot study for treatment of jumper’s knee. Injury 2009; 40: 598.
[8] Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med 2003; 33: 381-394.
[9] Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med 2009; 37: 2259-2272.
[10] Sanchez AR, Sheridan PJ, Kupp LI. Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 2003; 18: 93-103.
[11] Marx RE ; Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg 2004, 62: 489-496.
[12] Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med 2008; 1:165-174.
[13] Molloy T, Wang Y, Murrell GAC. The roles of growth factors in tendon and ligament healing. Sports Med 2003; 33: 381-394.
[14] Mehta S, Watson JT. Platelet rich concentrates: basic science and current clinical applications. J Orthop Trauma 2008; 22: 433-438.
[15] Pietrzak WS, Eppley BL. Platelet rich plasma: biology and new technology. J Craniofac Surg 2005; 16: 1043-1054.
[16] Bava ED, Barber FA. Platelet-rich plasma products in sports medicine. Phys Sports Med 2011; 39(3): 94-99.
[17] Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med 2011; 21(4): 344-352.
[18] Zhang J, Middleton KK, Fu FH, Im HJ, Wang JH. HGF mediates the anti-inflammatory effects of PRP on injured tendons. PLoS One 2013; 8(6): e67303.
[19] Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med 2010; 38(12): 2477-2486
[20] Geng Z, Wang C, Zhou H. Effect of platelet-rich plasma on tendon healing. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2011; 25(3): 344-348.
[21] Solchaga LA, Bendele A, Shah V, Snel LB, Kestler HK, Dines JS, et al. Comparison of the effect of intra-tendon applications of recombinant human platelet-derived growth factor-BB, plateletrich plasma, steroids in a rat Achilles tendon collagenase model. J Orthop Res 2013;12:23-34.
[22] Martinello T, Bronzini I, Perazzi A, Testoni S, De Benedictis GM, Negro A, et al. Effects of in vivo applications of peripheral bloodderived mesenchymal stromal cells (PB-MSCs) and platletrich plasma (PRP) on experimentally injured deep digital flexor tendons of sheep. J Orthop Res 2013; 31(2): 306-314.
[23] Uysal CA, Tobita M, Hyakusoku H, Mizuno H. Adipose-derived stem cells enhance primary tendon repair: biomechanical and immunohistochemical evaluation. J Plast Reconstr Aesthet Surg 2012; 65(12): 1712-1719.
[24] Sato D, Takahara M, Narita A, Yamakawa J, Hashimoto J, Ishikawa H, et al. Effect of platelet-rich plasma with fibrin matrix on healing of intrasynovial flexor tendons. J Hand Surg Am 2012; 37(7): 1356-1363.
[25] Chen L, Dong SW, Liu JP, Tao X, Tang KL, Xu JZ. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res 2012; 30(6): 991-997.
[26] Yuan T, Guo SC, Han P, Zhang CQ, Zeng BF. Applications of leukocyte- and platelet-rich plasma (L-PRP) in trauma surgery. Curr Pharm Biotechnol 2012; 13(7): 1173-1184.
[27] Maniscalco P, Gambera D, Lunati A, Vox G, Fossombroni V, Beretta R, et al. The “Cascade” membrane: a new PRP device for tendon ruptures. Description and case report on rotator cuff tendon. Acta Biomed 2008; 79(3): 223-226.
[28] Bosch G, Moleman M, Barneveld A, van Weeren PR, van Schie HT. The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand J Med Sci Sports 2013;8:22-26.
[29] Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int 2009; 30(11): 1101-1106.
[30] Lyras DN, Kazakos K, Agrogiannis G, Verettas D, Kokka A, Kiziridis G, et al. Experimental study of tendon healing early phase: is IGF-1 expression influenced by platelet rich plasma gel. Orthop Traumatol Surg Res 2010; 96(4): 381-387.
[31] Kajikawa Y, Morihara T, Sakamoto H, Matsuda K, Oshima Y, Yoshida A, et al. Platelet-rich plasma enhances the initial mobilization of circulation-derived cells for tendon healing. Cell Physiol 2008; 215(3):837-845.
[32] Aspenberg P. Stimulation of tendon repair: mechanical loading, GDFs and platelets. A mini-review. Int Orthop 2007; 31(6): 783-789.
[33] Bosch G, van Schie HT, de Groot MW, Cadby JA, van de Lest CH, Barneveld A, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: A placebo-controlled experimental study. J Orthop Res 2010; 28(2): 211-217.
[34] Virchenko O, Aspenberg P. How can one platelet injection after tendon injury lead to a stronger tendon after 4 weeks? Interplay between early regeneration and mechanical stimulation; Acta Orthop 2006; 77(5): 806-812.
[35] Hall MP, Ward JP, Cardone DA. Platelet rich placebo? Evidence for platelet rich plasma in the treatment of tendinopathy and augmentation of tendon repair. Bull Hosp Jt Dis 2013; 71(1): 54-59
[36] Kaux JF, Drion PV, Colige A, Pascon F, Libertiaux V, Hoffmann A, et al. Effects of platelet-rich plasma (PRP) on the healing of Achilles tendons of rats; Wound Repair Regen. 2012; 20(5): 748-756.
[37] Xiong X, Wu L, Xiang D, Ni G, Zhao P, Yu B. Effect of plateletrich plasma injection on early healing of Achilles tendon rupture in rats. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2012; 26(4): 466-471.
[38] Parafioriti A, Armiraglio E, Del Bianco S, Tibalt E, Oliva F, Berardi AC. Single injection of platelet-rich plasma in a rat Achilles tendon tear model. Muscles Ligaments Tendons J 2011; 1(2): 41-47.
[39] Schepull T, Kvist J, Norrman H, Trinks M, Berlin G, Aspenberg P. Autologous platelets have no effect on the healing of human Achilles tendon ruptures: a randomized single-blind study. Am J Sports Med 2011; 39(1): 38-47.
ment heading
10.1016/S2221-6189(14)60062-7
*Corresponding author: Hamid Karimi M.D., Member of ASPS, Associate Professor of Plastic Surgery, Faculty of Medicine, Iran University of medical Sciences, Tehran, Iran.
Tel : + 98 912 3179089
Fax: + 98 21 88770048
E-mail: hamidkarimi1381@yahoo.com; karimihamid11@gmail.com
 Journal of Acute Disease2014年4期
Journal of Acute Disease2014年4期
- Journal of Acute Disease的其它文章
- Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
- Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
- Epidemiological survey on scorpionism in Gotvand County, Southwestern Iran: an analysis of 1 067 patients
- The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
- Successful treatment of lower urinary tract obstruction with peritonealamniotic and vesicoamniotic shunting
- Simvastatin-induced Toxic Epidermal Necrolysis
