Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
Fatimah Lateef
Dept of Emergency Medicine, Singapore General Hospital, Singapore
Time-critical AMI Detection: A novel and fast technique using the 12-lead ECG
Fatimah Lateef*
Dept of Emergency Medicine, Singapore General Hospital, Singapore
Objective: To postulates a quick and relatively easy way to quantify the mass of viable myocardium in patients presenting with acute chest pain in the emergency department (ED).
Methods: The area under the QRS complex of a patient’s ECG was postulated to be proportional to the amount of viable myocardium. If a patient suffers a myocardial infarction and the myocardium loses its viability (i.e.the ability to fire or depolarise and repolarise), the area under the QRS complex was reduced. Results: By integrating the QRS complex of the ECG to obtain the area under the QRS complex, quantification of the mass of viable myocardium is carried out. The difference in size between the area before and after a patient has an acute coronary event, obtained from identical leads on both occasions, may advantageously show change in the mass of viable myocardium in the patient’s heart. Conclusions: This is a new method we are postulating and proofing the concept in this paper.
ARTICLE INFO
Article history:
Received 13 April 2014
Received in revised form 15 May 2014
Accepted 15 July 2014
Available online 20 November 2014 Keywords:
Myocardial infarction
Ischemia
QRS complex
Electrocardiography
Chest pain
Myocardial viability
1. Introduction
The electrocardiogram (ECG) is one of the most commonly done and readily available cardiovascular diagnostic tests. It is the test most often done for a patient with chest pain. The ECG is also used for monitoring patients in high dependency wards and intensive care units, for pre-operative screening and even in health screening assessments for individuals in certain high risk occupations[1-3].
Due to the wide applications of the ECG, the interpretation is also critical. These days, with advances in technology, electronic and computerised systems are more readily available for storage as well as interpretation of ECGs. Patients previous ECGs stored in the database or electronic archive systems, serve as a valuable resource as baseline comparison to the current ECG and can play a role in clinical decision making and management of the patient with acute chest presentations in the Emergency Department (ED)[1,2].
There are many causes for the acute chest pain that makes a person presents to the ED. Causes include both cardiac and non-cardiac etiologies. Some examples of the latter include, oesophageal spasm, gastric pain, pleuritic chest pain, costo-chondritis and musculo-skeletal chest pain. The ECG has been used for decades to help differentiate between both types of etiologies, but we do know now that this is not always possible.
There are patients with acute coronary syndrome who do not manifest any overt ECG changes. There are also patients whose ECG changes were not present on the first presenting ECG, but developed much later on and was detected by the performance of serial ECGs or continuous ST-segment trend monitoring. The 12-lead ECG does not always show overt changes to diagnose acute coronary syndrome (ACS)[4-7].
Coronary artery disease (CAD) remains an important cause of morbidity and mortality worldwide[1,4,5,7]. Manypatients with underlying CAD and heart failure may have a combination of both viable and non-viable myocardium. Many are now beginning to realise that it is important and relevant to be able to differentiate viable from non viable myocardium[8-10]. However the picture is complicated because of the phenomenon of myocardial stunning (where the viable myocardium is non contractile or dysfunctional in contraction, for a variable period of time, but is reversible, often after reperfusion) and myocardial hibernation (ischaemic myocardium , usually supplied by a narrowed coronary vessel, in which these ischaemic myocardial cells continue to remain viable , but contraction is chronically depressed)[8,11-13].
This paper postulates a quick and relatively easy way to quantify the mass of viable myocardium in patients presenting with acute chest pain in the ED. This is done by integrating the QRS complex of an ECG to obtain the area under the QRS complex, which can quantify the mass of viable myocardium.
The quantifiable difference in the size of the area of the QRS complex of the current ECG, compared to an earlier ECG obtained from the same patient on a prior occasion may show change in the mass (gain or loss) of viable myocardium in the patient’s heart over a period of time.
Therefore if there is a loss or decrease in the mass of viable myocardium, there could possibly be a new ischaemic event such as ACS or infarction, that has taken place between the time the two ECGs were done. The comparison of the area under the QRS complex can be assessed either visually or quantitatively and the comparison must be done using identical leads.
2. Methodology
The area under the QRS complex of a patient’s ECG is postulated to be proportional to the amount of viable myocardium. If a patient suffers a myocardial infarction and the myocardium loses its viability (i.e. the ability to‘fire’ or depolarise and repolarise), the area under the QRS complex is reduced. By comparing the areas under the QRS complexes at two different times (e.g.T=0 and T=1) for the same patient, a reduction of the area represents a loss of the viable myocardium. Determining the difference may be done either visually or quantitatively (Figure 1).
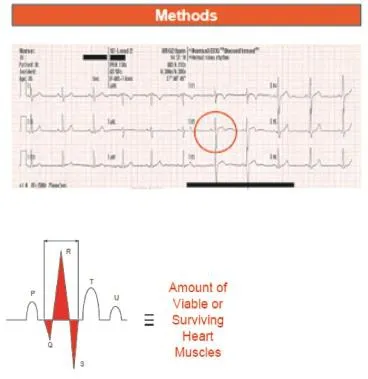
Figure 1. Area under the curve of the QRS complex.
3. Results
In a retrospective analysis of 42 ECG tracings belonging to 10 patients with acute myocardial infarction, with a mean age of 64 years and diagnosed by board certified cardiologists to have sustained ischaemic insults, visual inspection of the area under the QRS complex, obtained by utilising lead V2, showed that the areas on the follow up ECGs have all been reduced by varying degrees. Further calculation performed showed the reduction, on average to be 36.7% (Max: -83.9%, Min: -1.3%) (Figure 2)
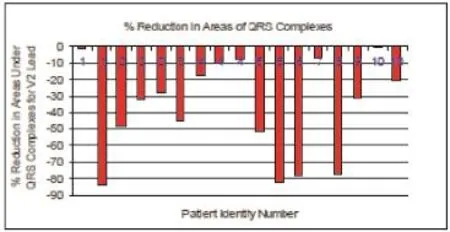
Figure 2. Percentage of reduction of the area under the QRS complex after AMI.
4. Discussion
This novel method utilises the fact that viable myocardialcells will contribute towards cardiac contraction. Myocardial viability is a binary phenomenon: segments of the myocardium or heart muscles are either viable or not because of the all or none property of an excitable cell. The QRS complex is recorded on the ECG tracing when the heart is undergoing ventricular depolarization. When the myocardial cells have suffered an ischaemic insult, they become non-viable and thus cannot ‘fire’ (i.e.depolarise and repolarise) and hence this is postulated to affect the appearance and manifestation/morphology of the QRS complex.
With the advent and more widespread use of digital ECGs, it may be possible to consider incorporating this capability into the machine software and technology. Further work will be required to ensure a more robust validation technique and corroboration of the results in a larger cohort study.
This novel technique proposed shows high potential for practical application and more work in the form of prospective validation. Software trial in a larger cohort could help prove convincingly that the area under the QRS complex of the ECG reflects myocardial viability and any ischaemic insults/infarction will result in a reduction of the area of a subsequent ECG in any patient.
It will also be useful in the future study to correlate this with other parameters which are currently used to assess viability, such as the calculated ejection fraction or MIBI scan findings[12-14]. This novel proposed method provides an option to be used in time-critical AMI diagnosis, when ECG changes are less clear, history is not suggestive or in silent AMI, whilst waiting for the cardiac markers to come back from the laboratory.
Conflict of interest statement
We declare that we have no conflict of interest
[1] Fesmire FM, Percy RF, Bardour JB, Wharton DR, Calhoun FB. Usefulness of automated serial 12-lead ECG monitoring during the initial Emergency Department evaluation of the patient with chest pain. Ann Emerg Med 1996; 31(1): 3-11.
[2] O’Connor DP. Knoblaunch MA. ECG testing during athletic preparticipation physical examination. J Athl Train 2010; 45(3); 265-272.
[3] E F van der Wall. The exercise ECG: still a useful exercise? Neth Heart J 2009; 17(2): 47-49.
[4] Gibler WB, Cannon CP, Blomkalns AL, Char DM, Drew BJ, Hollander JE, et al. Practical implementation of the Guidelines for Unstable Angina/Non-ST-Segment Elevation Myocardial Infarction in the emergency department. Ann Emerg Med 2005; 46: 185.
[5] Pollack CV Jr, Diercks DB, Roe MT, Peterson ED, American College of Cardiology, American Heart Association. 2004 American College of Cardiology/American Heart Association guidelines for the management of patients with ST-elevation myocardial infarction: implications for emergency department practice. Ann Emerg Med 2005; 45: 363-365.
[6] Graber MA, Darby-Steward A, Dach R. Can ECG rule out acs if performed while the patient is having chest pain? Am Fam Physician 2010; 82(10): 1185-1186.
[7] Jacobson C. ECG Challenges: Diagnosis of acute coronary syndrome. AACN Advanced Critical Care 2008; 19(1): 101-108.
[8] Conti CR. The stunned and hibernating myocardium: a brief review. Clin Cardiol 1991; 14(9): 708-712.
[9] Shabana A, El-Menyan A. Myocardial viability: what we know and what is new? Review Article. Cardiol Res Practice 2012.
[10] Camici PE, Prasad SK, Rimoldi OE. Stunning, hibernation and assessment of myocardial viability. Contemporary reviews in cardiovascular med. Circulation 2008; 117: 103-114.
[11] Al-Mohammad A, Norton MY, Malig IR, Patel JC, Welch AE, Mikecz P, et al. Can the surface ECG be used to predict myocardial viability. Heart 1999; 82: 663-667.
[12] Kataoka H, Madias JE. Changes in the amplitude of ECG QRS complex during follow up of heart failure patient. J Electrocardiol 2011; 44(3): 394.
[13] Okin PM, Rowan MJ, Devereux RB, Pickering TG, Borer JS, Kligfield P, et al. Time Voltage QRS area of the 12 lead ECG. Hypertension 1998; 31: 937-942.
[14] Cease KB, NIcklas JM. Prediction of LV ejection fraction using simple quantitative clinical information. Am J Med 1986; 81: 429-436.
ment heading
10.1016/S2221-6189(14)60065-2
*Corresponding author: Fatimah Lateef, Dept of Emergency Medicine, Singapore General Hospital, Singapore. Tel: 65 63213558/4972
E-mail: fatimah.abd.lateef@sgh.com.sg
 Journal of Acute Disease2014年4期
Journal of Acute Disease2014年4期
- Journal of Acute Disease的其它文章
- Acute and sub-acute toxicity study of Clerodendrum inerme, Jasminum mesnyi Hance and Callistemon citrinus
- Different profiles of mental and physical health and stress hormone response in women victims of intimate partner violence
- Epidemiological survey on scorpionism in Gotvand County, Southwestern Iran: an analysis of 1 067 patients
- The acute effect of the antioxidant drug “U-74389G” on red blood cells levels during hypoxia reoxygenation injury in rats
- Successful treatment of lower urinary tract obstruction with peritonealamniotic and vesicoamniotic shunting
- Simvastatin-induced Toxic Epidermal Necrolysis
