Combination of percutaneous radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: observation of clinical effects
Department of Hepatobiliary Surgery, The First Affliated Hospital of Bengbu Medical College, Bengbu 233004, China
Correspondence to: Hui-Chun Liu, Professor. Department of Hepatobiliary Surgery, The First Affliated Hospital of Bengbu Medical College, 287 Chang Huai Road, Bengbu 233004, China. Email: liuhuichun2013@163.com.
Combination of percutaneous radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: observation of clinical effects
Hui-Chun Liu, Er-Bo Shan, Lei Zhou, Hao Jin, Pei-Yuan Cui, Yi Tan, Yi-Min Lu
Department of Hepatobiliary Surgery, The First Affliated Hospital of Bengbu Medical College, Bengbu 233004, China
Correspondence to: Hui-Chun Liu, Professor. Department of Hepatobiliary Surgery, The First Affliated Hospital of Bengbu Medical College, 287 Chang Huai Road, Bengbu 233004, China. Email: liuhuichun2013@163.com.
Objective:To observe the clinical effect of radiofrequency ablation (RFA) combined with transcatheter arterial chemoembolization (TACE) for advanced hepatocellular carcinoma (HCC).
Methods:A total of 92 cases of advanced primary liver cancer underwent TACE and RFA treatment from June 2005 to 2011 at the Department of Hepatobiliary Surgery, the First Affiliated Hospital of Bengbu Medical College. A total of 88 cases with complete clinical treatment and follow-up data were divided into two groups: 43 patients treated with TACE (TACE group) and 45 patients that received TACE combined with RFA treatment (TACE + RFA group). After clinical data assessment, tumor size and survival status were not signifcantly different between the groups as determined by stratifed analysis.
Results:Before and after surgery, spiral CT radiography and color comparison observed ablation conditions. The tumor necrosis rates after treatment (CR + PR) were 67.4% (29/43) and 91.1% (41/45) for the TACE and combined treatment groups, respectively, and the difference was statistically signifcant (P<0.05). The quality of life was significantly improved for patients undergoing TACE + RFA compared with the control group. Survival duration was not signifcantly different in patients undergoing TACE + RFA compared with the control group.
Conclusions:In this study, the effect of RFA combined with TACE treatment was better than TACE alone in treating advanced HCC.
Liver cancer; radiofrequency ablation (RFA); transcatheter arterial chemoembolization (TACE); quality of life; survival period
View this article at:http://dx.doi.org/10.3978/j.issn.1000-9604.2014.08.18
Introduction
Recent evidence shows that there are 740,000 new cases and 690,000 deaths related to liver cancer around the world each year; among them more than 50% are in China, with liver cancer being the second leading cause of death by malignant tumor in the country (1). Unfortunately, diagnosis of middle-late stage disease is very common, and such advanced cancer is inoperable. For unresectable liver cancer, we often use palliative methods including transcatheter artery embolization (TACE) chemotherapy, portal vein embolism chemotherapy (PVCE), radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and microwave coagulation therapy (PMC) for clinical treatment.
Currently, all single treatment methods have specific limitations and the long-term effect is not ideal; however, comprehensive treatment can often enhance the curative effect. Both TACE and RFA have definite benefits in curing liver cancer, are minimally invasive, and have a high application value, but are limited when used as separate lone therapies. TACE has emerged as the frst choice in treating unresectable liver cancer, although its tumor necrosis rate is low, and the curative effect is unsatisfactory. Since the1990s, RFA has been extensively used in China and abroad and has proven to be effective in treating liver cancer (2,3). TACE combined with RFA can enhance individual treatment advantages and amplify the effect in cooperation, which reflects the principle of comprehensive treatment to advanced hepatocellular carcinoma (HCC). TACE with RFA can improve curative effect in treatment of HCC, as reported in China and other countries (4,5).

Table 1 Comparison of group characteristics
Comparing clinical data between patients with middlelate stage HCC that received simple TACE treatment and those that received TACE combined with RFA, we provide evidence of enhanced curative effects in patients with HCC when given the combined treatment regimen.
Methods
Clinical information
Patients with middle-late stage primary carcinoma of the liver who could or should not be accepted for surgical treatment, according with the AASLD diagnostic criteria, were included in clinical studies from June 2005 to June 2011 in our department. AASLD diagnostic criteria: (I) tumor marker detection and (or) pathology through imaging (CT/MRI/US) in accord with diagnosis of primary liver cancer; (II) imaging revealed the following general pathology type: Nodular HCC (3< diameter <5 cm, single or multiple), block type liver cancer (3< diameter <5 cm, single or multiple), giant block type liver cancer (diameter >10 cm), incorporation with a single carcinoma bolt in a nonmain portal vein, combined with extrahepatic metastases (including hepatic portal and lymph node metastasis), but through evaluation can be effectively controlled by RFA or radiation therapy; (III) whose liver function is child A or B level though cannot undergo surgery with TACE combined RFA therapy in the past, or liver function is child C and is improving through the symptomatic treatment. Altogether, the study involved 88 patients that accorded with diagnosis standards and were followed-up. Before treatment all patients or their relatives were informed of the various treatments that may produce beneft and risks, and individual expenditure. According to the methodology used to group patients, 88 patients were divided into two groups: RFA group and control group. The latter had 43 cases, receiving only TACE and symptomatic treatment; the RFA group had 45 cases, treated by conventional TACE followed by RFA and symptomatic treatment. All patients had no or minimal ascites, no bleeding tendency, and normal coagulation function and routine blood. The treatment and control groups were comparable. Patients’ characteristics are shown in Table 1 (controlling Barcelona stage).
Treatment methods for treatment group (RFA + TACE + symptomatic treatment)
We performed conventional TACE and symptomatic treatment before RFA, utilizing the iodine oil accumulation effect to decide the RFA treatment time. If iodine oil accumulation was poor, the patients received RFA treatment as early as possible; if iodine oil accumulation was good, the patients received RFA until the tumor size further narrowed. A radiofrequency generator (Sichuan Mianyang Khalid LDRF–a 120 s type radio frequency therapeutic apparatus with a working frequency of 400 KHz,) and electrode needle (match LDRF–120 s multipolar RFA electrode needle) were used in the treatment paradigms.
Under ultrasound guidance, the most optimal puncture site was selected and marked. After local anesthesia infiltration, multipolar electrode needle puncture into the tumor was performed and radio frequency treatment wasdeployed with a 1 cm safety margin, paying close attention to avoid large blood vessels and the intrahepatic bile duct. Scattered tumors with a size less than 5 cm received onetime ablation for 5-10 min with the electrode penetrating to the center of tumor. If the tumor size exceeded 5 cm and had a rich blood supply through relevant examination, it was necessary to perform TACE before RFA. During treatment, we utilized ultrasound to monitor echo changes in the treatment area and estimated a treatment range with super echo group size. If necessary, we adjusted the electrode position for multi-point therapy until the entire tumor echo was enhanced. Finally, we performed needle tract cauterization to stop bleeding and prevent tract seeding.
Control trial (TACE and symptomatic treatment)
All patients received TACE and symptomatic treatment and further treatment measures were determined according to lipiodol deposition. If a patient’s lipiodol deposition was acceptable, only regular follow-up treatment was required. If deposition was unsatisfactory, TACE treatment continued another day.
Postoperative observation and Auxiliary treatment
One month after the operation, we started follow-up patient observation. Patient observation lasted 6 months. Observation indices: (I) survival quality: the pain frequency (time/24 hours) VAS table was used to evaluate degree of pain, weight change, and daily activities; (II) liver function monitoring: 5-7 days after treatment and discharge from hospital, respectively, liver function biochemical index and observation of clinical manifestations (ascites, jaundice, etc.) were monitored. According to the 2007 American FDA guidelines for hepatotoxicity evaluation, the defnition of liver damage are as follows: (i) the ALT and AST elevated greater than 3 times normal; (ii) transaminase increased less than 3 times normal, with simultaneous total bilirubin elevation twice normal levels (excluding bile duct obstruction); (iii) exclusion of other reasons (viral hepatitis and liver toxicity caused by drugs, etc.) for elevation of aminotransferase and bilirubin; (III) survival time: Kaplan-Meier method for calculation of cumulative survival rate, COX proportional risk model analysis were used for analyzing treatment effects on survival and a chi-square test was used to compare differences between groups, with a P<0.05 considered statistically significant (SPSS version 17.0); (IV) tumor damage monitoring: 1 month after the operation, patients were reviewed via CT or enhanced ultrasound and tumor markers. If no pathological enhancement was detected internally or at ablation edges, all tumor lesions disappeared after treatment with no new lesions, or tumor markers declined to normal and maintained for 4 weeks, we defned these individuals as in“complete remission (CR)”. If preoperative AFP was higher than normal, but no significant decline or even elevation was observed postoperatively, imaging documented increased necrosis, and partial lesion enhancement remained at the tumor edge or internally, we defined this as “partial remission (PR)”. Patients in PR received further TACE to the necrotic part of the tumor. Six months after treatment, reviewing tumor markers monthly via ultrasound or computed tomography (CT) imaging, some patients may require strengthened MR inspection. If so, every 3-4 months, tumor markers and CT were evaluated to monitor the status of radio frequency ablation for local recurrence of the tumor, new intrahepatic lesions and extrahepatic metastases. If any residual lesions or recurrence are revealed on examination, patients received immediate RFA or other treatment, followed-up with the same symptomatic auxiliary treatment.
Results
Survival quality and liver function changes
Four months after the operation, pain frequency and extent in the treatment group was considerably reduced or alleviated compared to the control group. Nine of 45 cases in the treatment group exhibited 20% weight loss, and 15 patients in the control group (out of 43 patients) had lost 79.1% of their starting weight. Liver function in the treatment group was noticeably improved compared to the control group. A total of 16 patients in the control group presented with ascites at 4 months, and 8 in the treatment group. Four months post-operatively, upper gastrointestinal hemorrhage was observed in nine and four patients in the control and treatment groups, respectively, as shown in Table 2.
Survival time
From Table 3 and Figure 1, respectively, the results show that the treatment group’s average survival time was 37.80 vs. 30.45 months for the control, group. The 5-month survival rate of the treatment group was notably higher than the control group. However, the overall survival rateshowed no statistical significance (Table 4, P=0.081 or>0.05). These fndings were likely due to a low number of cases and follow-up time may not have been long enough.
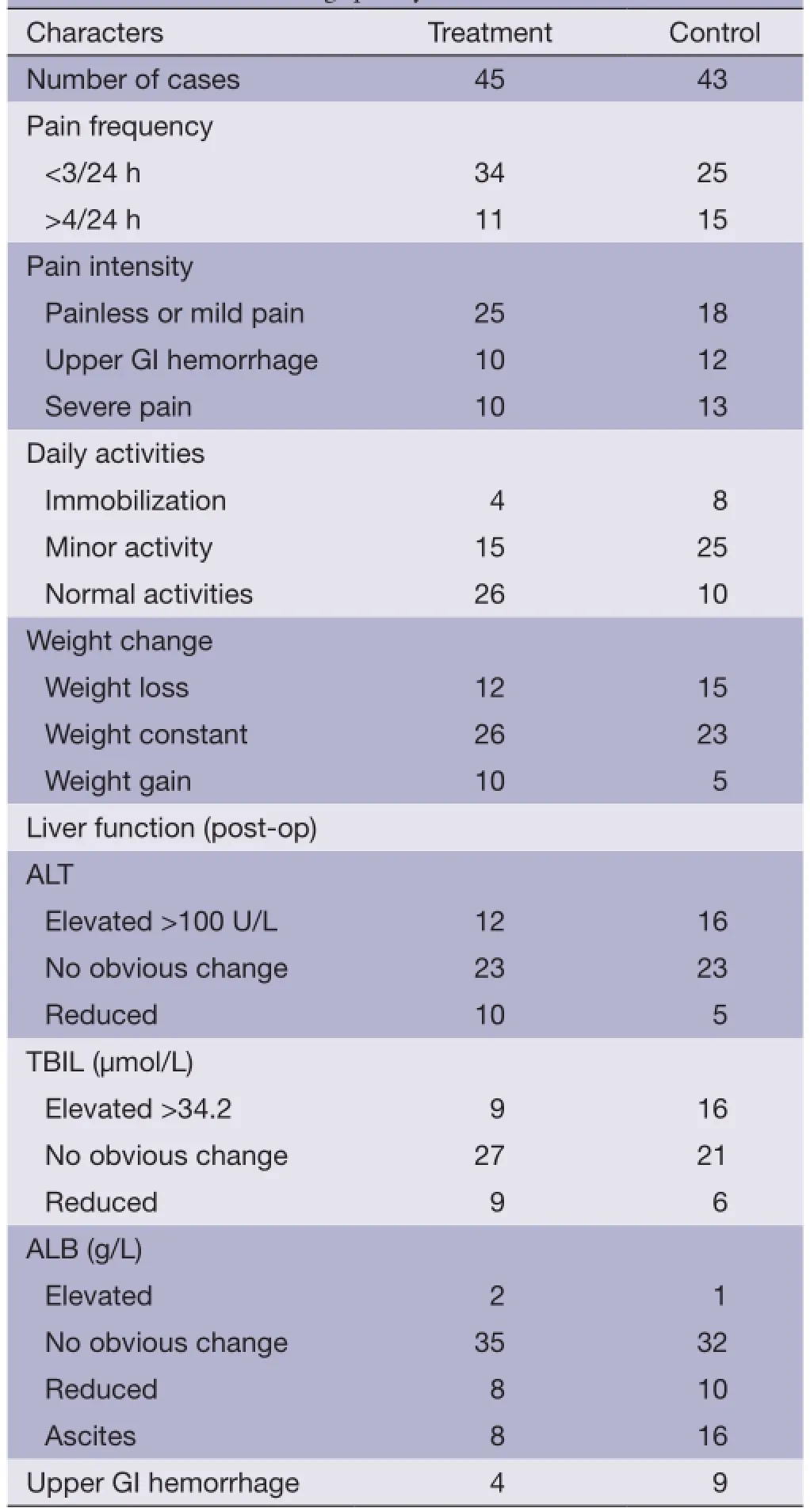
Table 2 Contrast in living quality
Tumor damage
About 1-1.5 months after treatment, the two groups had follow-up CT and DSA evaluations. The total effective rates (CR + PR) of tumor necrosis in the two groups were 67.4% (29/43) and 91.1% (41/45), in the control and treatment groups, respectively, but this difference was not statistically significant (P<0.05). The complete necrosis rates of the simple TACE group (CR) 27.9% (12/43) and the combination therapy group (CR) 83.2% (37/45) were statistically signifcantly different (P<0.05).
Complications
The most common complications in liver damage were symptoms of abnormal biochemical index fuctuations, icteric presentation, and ascites after all 175 TACE treatments. Twenty cases (11.4%), which conforming to the liver function damage standards, required necessary measures for liver protection and symptomatic treatment. However, eight patients (6.2%) conformed to the liver function damage standard after all 125 RFA treatments. Transient abdominal pain, fever, gastrointestinal symptoms were common side effects of TACE treatments; however, all patients improved after symptomatic treatments. Common side effects from RFA therapy were abdominal pain, fever, and gastrointestinal adverse reaction, improving after symptomatic treatments, but planting metastasis was not present.
Discussion
With the improvement of RFA technology, standardizedtreatment and highly skilled application, large liver cancer inactivation rates have improved. At present, RFA is not suffciently effective against larger tumors, and recurrence rates for large liver tumors are high. The technique is minimally invasive, relatively safe and its advantages are great enough that an agreement to acquire large multicenter clinical research support has been reached. Using RFA as local treatment for tumors with a diameter of 3 cm or less, a large amount of evidence presently suggests that the HCC curative effect is equal to surgical resection (6). Concerning RFA for HCC, resulting tissue necrosis effects are directly related to focal size. Multiple electrode RFA needles can induce coagulation and tumor degradation, and this treatment compared with other local therapies has a higher complete tumor necrosis rate, low intrahepatic recurrence rate, requires fewer treatments, and results in longer patient survival (7).
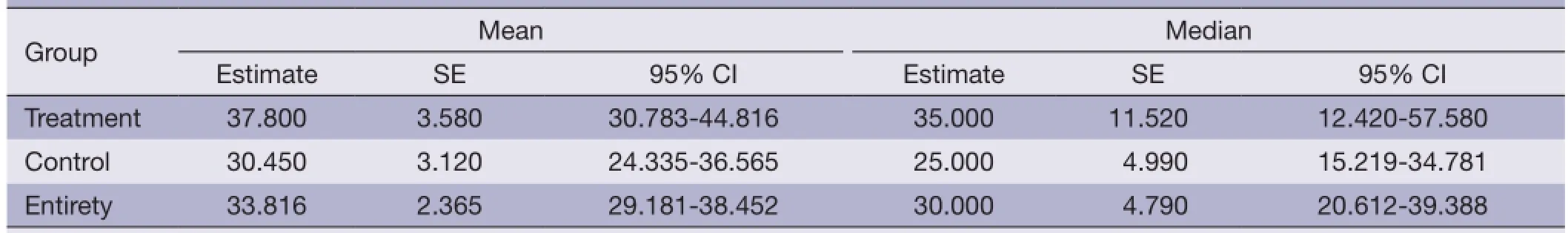
Table 3 Mean and median of survival table
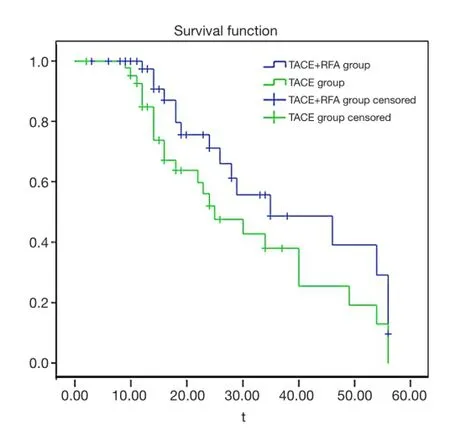
Figure 1 Patient survival curve. Survival time of treatment group (TACE + RFA group) were higher than that of control group (TACE group). TACE, transcatheter arterial chemoembolization; RFA, radiofrequency ablation.
Research shows that RFA therapy also importantly improves the immune ability of the antineoplastic (8,9). The main mechanisms are: (I) inactivation of tumor cells diminishes immune system inhibition; (II) the improvement of patients’ general condition promotes recovery of immune function; (III) RFA radiates considerable heat locally, and may enhance tumor cell surface antigen exposure improving the antigenicity of the tumor; (IV) after heat treatment the tumor can synthesize a kind of irritability protein–heat shock protein HSP70, that enhances tumor specific antigen presentation and stimulates the body’s immune system. However, RFA has limitations including range of effcacy for single treatments, tumors greater than 3 cm diameter easily produces residual lesions, blood fowing to the tumor will dissipate most of the heat, and confnes of the range of coagulation necrosis. Furthermore, TACE, indirectly through embolism in tumor blood vessels and chemotherapy drug effects, cannot induce necrosis of all middle-late stage liver cancer cells in a single treatment and can only limit growth. Soon, new tumor angiogenesis begins, making it diffcult for complete eradication of the tumor. Multiple TACE treatments can damage normal liver tissue, impair liver function and even induce liver failure.
RFA and TACE therapies alone clearly have certain limitations in treating late stage liver cancer, but from respective outcome characteristics, they can be complementary. Studies have shown that TACE and RFA combination therapy of mid- and late-stage large liver cancer can improve ablation effect (10-13). Combined with TACE, RFA can effectively reduce lesion blood supply, minimizing blood flow heat dissipation and enhancing centralized thermal effects for complete focal necrosis. This can expand the scope of each ablation treatment. For larger tumors, although multiple thermal ablations can be applied, incomplete focal necrosis is a common outcome. Therefore, TACE therapy may be useful prior to preoperative RFA for single large HCC (>3.5 CRN). TACE can improve blockade of tumor blood supply, and combination with chemotherapy may help reduce tumor size to improve RFA effects on minimizing tumor residue. For multiple carcinomas, TACE can also locatesmall lesions that imaging examination cannot increase opportunity for early treatment.

Table 4 COX model regression variable scale of treatment effects on survival rates of advanced liver cancer patients
Since the start of the RFA project in 2004, preliminary results from the gradual treatment of advanced liver cancer patients by palliative therapy with RFA indicated improvement over TACE and chemotherapy. Our experience showed that RFA is particularly suitable for advanced liver cancer patients with associated severe cirrhosis and abnormal liver function. Elevated temperature produced by RFA treatment sensitizes cancer cells to chemotherapy, and TACE treatment times can be reduced, decreasing chemotherapy damage to the liver. The biggest advantage of this therapy is the obvious improvement in patients’ quality and quantity of life. In addition, there were no apparent adverse effects. The five patients with tumor diameter greater than 10 cm (max, 15 cm) were treated with RFA after TACE and exhibited decreased tumor volume. Postoperative symptom improvement was particularly notable. We believe it is critical to maximize reduction of tumor load for patients with advanced liver cancer to improve living quality and prolong survival.
TACE combined with RFA can control internal liver lesions as well as extrahepatic metastases. Controlling metastases through RFA (especially in lung metastases), can achieve a complete necrosis “resection” effect, reducing body tumor load and positively influencing survival prognosis. TACE is more known for improving liver function than RFA. However, TACE followed by RFA can reduce the number of TACE treatments while delivering a similar outcome. This is beneficial for middle and latestage HCC with poor liver function, potentially enhancing overall survival in these patients. However, this conclusion still requires further investigation.
Conclusions
This study compared simple TACE with RFA and TACE combination treatment in two groups of patients. Outcomes evaluated included tumor damage, survival, and the combination therapy resulted in obvious improvement in alpha-fetoprotein decline, local tumor necrosis and quality and duration of life. This treatment effect is clearly superior to simple embolism chemotherapy. Along with the progress of modern medicine and medical means, treatment of malignant liver cancer has improved. Although comprehensive treatment is increasingly accepted, a large clinical trial is necessary to assess curative effects. Individual factors should also be fully considered for selecting the best sequential combination treatments.
Acknowledgements
Natural Science Research Program of Education Bureau of Anhui Province (No. J2009A163).
Disclosure: The authors declare no confict of interest.
1. Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90.
2. Lencioni R, Cioni D, Crocetti L, et al. Early-stage hepatocellular carcinoma in patients with cirrhosis: longterm results of percutaneous image-guided radiofrequency ablation. Radiology 2005;234:961-7.
3. Chen MS, Li JQ, Liang HH, et al. Comparison of effects of percutaneous radiofrequency ablation and surgical resection on small hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi 2005;85:80-3.
4. Liao GS, Yu CY, Shih ML, et al. Radiofrequency ablation after transarterial embolization as therapy for patients with unresectable hepatocellular carcinoma. Eur J Surg Oncol 2008;34:61-6.
5. Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci 2012;57:3026-31.
6. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation as frst-line treatment for small solitary hepatocellular carcinoma: long-term results. Eur J Surg Oncol 2010;36:1054-60.
7. Germani G, Pleguezuelo M, Gurusamy K, et al. Clinical outcomes of radiofrequency ablation, percutaneous alcohol and acetic acid injection for hepatocelullar carcinoma: a meta-analysis. J Hepatol 2010;52:380-8.
8. Nobuoka D, Motomura Y, Shirakawa H, et al. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specifc cytotoxic T lymphocytes. Int J Oncol 2012;40:63-70.
9. Zerbini A, Pilli M, Fagnoni F, et al. Increased immunostimulatory activity conferred to antigenpresenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 2008;31:271-82.
10. Lu Z, Wen F, Guo Q, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: a meta-analysis ofrandomized-controlled trials. Eur J Gastroenterol Hepatol 2013;25:187-94.
11. Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 2013;31:426-32.
12. Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol 2006;16:661-9.
13. Sugimori K, Nozawa A, Morimoto M, et al. Extension of radiofrequency ablation of the liver by transcatheter arterial embolization with iodized oil and gelatin sponge: results in a pig model. J Vasc Interv Radiol 2005;16:849-56.
Cite this article as:Liu HC, Shan EB, Zhou L, Jin H, Cui PY, Tan Y, Lu YM. Combination of percutaneous radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: observation of clinical effects. Chin J Cancer Res 2014;26(4):471-477. doi: 10.3978/j.issn.1000-9604.2014.08.18
10.3978/j.issn.1000-9604.2014.08.18
Submitted Apr 02, 2014. Accepted for publication Jul 22, 2014.
 Chinese Journal of Cancer Research2014年4期
Chinese Journal of Cancer Research2014年4期
- Chinese Journal of Cancer Research的其它文章
- Aberrant DNA methyltransferase 1 expression in clear cell renal cell carcinoma development and progression
- In vitro effect of iASPP on cell growth of oral tongue squamous cell carcinoma
- Long-term survival outcomes of video-assisted thoracic surgery for patients with non-small cell lung cancer
- Embolization of symptomatic renal angiomyolipoma with a mixture of lipiodol and PVA, a mid-term result
- Decline of serum CA724 as a probable predictive factor for tumor response during chemotherapy of advanced gastric carcinoma
- TPX2 knockdown suppressed hepatocellular carcinoma cell invasion via inactivating AKT signaling and inhibiting MMP2 and MMP9 expression
