A path for diagnosis and therapy of colon cancer: a continuous quality improvement
Jacopo Giuliani, Marina Marzola
1Department of Medical Oncology, Mater Salutis Hospital, Legnago (VR) 37045, Italy;2Clinical Oncology Unit, St. Anna University-Hospital, Ferrara 44100, Italy
Correspondence to: Dr. Jacopo Giuliani. Department of Oncology, Mater Salutis Hospital, ASL 21 della Regione Veneto, Legnago (VR) 37045, Italy. Email: giuliani.jacopo@alice.it.
A path for diagnosis and therapy of colon cancer: a continuous quality improvement
Jacopo Giuliani1, Marina Marzola2
1Department of Medical Oncology, Mater Salutis Hospital, Legnago (VR) 37045, Italy;2Clinical Oncology Unit, St. Anna University-Hospital, Ferrara 44100, Italy
Correspondence to: Dr. Jacopo Giuliani. Department of Oncology, Mater Salutis Hospital, ASL 21 della Regione Veneto, Legnago (VR) 37045, Italy. Email: giuliani.jacopo@alice.it.
Objective:Prognosis of colorectal cancer strongly depends on stage at diagnosis, which can be cured in most cases at an early stage. The results were supported by different screening programmes. Few data concerning analysis of different phases of Colorectal Cancer Program were reported in literature. The aim of this study is to analyze “step by step”, from a longitudinal type, the Colorectal Cancer Program, active at our Institution, verifying compliance with standards of care.
Methods:We compared two different populations during the same period: patients referring to our Clinical Oncology Unit coming from Regional Colorectal Cancer Screening Program and the other population that was not in any Colorectal Cancer Program.
Results:Considering patients from the Regional Colorectal Cancer Screening Program (19 patients, corresponding to 24.0% of the general case study), 3 (15.8%) were deceased and 16 (84.2%) were alive without evidence of the disease (NED). Concerning patients that are not coming from Regional Colorectal Cancer Screening Program (61 patients, corresponding to 76.0% of the general case study), 9 (14.8%) were deceased, 43 (70.5%) were NED, 8 (13.1%) were alive with metastases and 1 (1.6%) was lost during follow-up (PFU).
Conclusions:On the basis of this experience, we concluded for high-quality care for both populations. Any critical point should be carefully analyzed in order to implement quality of care.
Colorectal cancer screening; colorectal cancer program; quality of care
View this article at:http://dx.doi.org/10.3978/j.issn.1000-9604.2014.12.08
Introduction
Colorectal cancer is the second most common cancer cause of death in Europe, accounting for more than 200,000 deaths per year. Prognosis strongly depends on stage at diagnosis and the disease can be cured in most cases if diagnosed at an early stage (1). These results were supported by different screening programmes (2,3). The creation of the Path Diagnostic and Therapeutic Care (PDTA), established in multiprofessional and multidisciplinary perspective, should deliver the documents to be able to outline the organizational structure and quality standards that are reflective of the scope of care, feasible to implement and supported by evidence. Their adoption should allow not only efficiency but also effectiveness and homogeneity of intervention by reducing the waiting time and simultaneously the passive mobility. The PDTA is therefore a tool for both clinical and organizational governance processes inside a hospital (Clinical Governance), which improve and make more easily accessible path to the person facing the disease (4). Few data concerning analysis of the different phases of the PDTA were reported in literature: the goal is to maintain and improve the quality of care. The aim of this study is to analyze the so called “Colorectal Cancer Program” or PDTA active at our Institution for patients with suspected colon cancer. Our purpose is to analyze “step by step”, from a longitudinal type, the PDTA in patients with colon cancer (active at the St. Anna University-Hospital of Ferrara fromApril 2005), verifying compliance with these standards and comparing the results for patients for whom the PDTA was institutionalized (patients coming from the Regional Colorectal Cancer Screening Program) with the results of all other patients underwent surgical resection for colon cancer and referred to our Clinical Oncology Unit at the same time-period in order to verify all possible differences in the quality of service offered to each other and, where necessary, to propose amendments to implement the results.
Materials and methods
All consecutive patients with colorectal cancer referred to Clinical Oncology Unit of St. Anna University-Hospital of Ferrara (Italy) between 01/01/2009 and 31/12/2010 and resident into the district of the city of Ferrara were retrospectively analyzed. For these data, it was started crossing the information retrieved from the archive computerized of our Clinical Oncology Unit (compiled from July 1974 and updated February 2011) with data provided by the Institute of Pathology for positive histological examination for “colon cancer” carried on“specimen” in the years 2009-2010. Of those patients, the residence was then searched through computerized system ISESWeb. All information was obtained from case history and patient’s medical history (from hospital records only).
The inclusion criteria comprised: (I) patients with colon cancer; (II) patients referred to Clinical Oncology Unit of St. Anna University Hospital of Ferrara between 01/01/2009 and 31/12/2010; (III) patients underwent surgery for colon cancer c/o St. Anna University Hospital of Ferrara; patients with histologically positive for “colon cancer” carried on “surgical specimen” in 2009-2010; and (IV) patients residing c/o the Urban Area of the City of Ferrara. The exclusion criteria comprised: (I) the presence of rectal lesion location; and (II) patients age >80 years.
The data were transcribed on paper ballots and then fed into a computer database. Native to the variables, we have added other derived and aggregated to facilitate statistical analysis. Data were expressed as absolute values or percentages or x±s. First we performed an overall assessment of the data collected according to the clinicopathological features of patients. Subsequently a detailed analysis of all the different phases of the course was made, comparing the results achieved with optimal standards (taking into account the international literature and national and regional reference documents) (5,6) and proposing measures for implementation of results, where necessary.
The main indicators for the diagnostic phase in the study are summarized below: (I) all patients referred by the Regional Colorectal Cancer Screening Program who have positive colonoscopy for colon cancer should be borne by the Day Hospital (DH) Medical Gastroenterology for the continuation of the diagnostic staging and treatment (Numerator = number of patients with positive colonscopy for colon cancer afferent from Regional Colorectal Cancer Screening Program that have been taken over by the DH Medical Gastroenterology for the continuation of the diagnostic staging and treatment, Denominator = number of patients with positive colonscopy for colon cancer afferent from Regional Colorectal Cancer Screening Program). Standard: >90%; and (II) within 7 d will be completed by the DH Medical Gastroenterology all exam needed for surgery (Numerator = number of patients referred to the Colon Cancer Program where preoperative tests were performed within 7 d by the DH Medical Gastroenterology; Denominator = number of patients who were referred to the Colon Cancer Program). Standard: >90%.
The main indicators for the surgical phase in the study are summarized below: (I) the waiting time between diagnosis (inclusion in the list) and hospitalization for surgery should not exceed 30 d (Numerator = number of patients with colon cancer who underwent surgery within 30 d of diagnosis, Denominator = number of patients with colon cancer underwent surgery). Standard: >90%; (II) the radicality of resection must be confirmed by both intraoperative judgment (no macroscopically visible residues) and the subsequent histological examination (Numerator = number of patients underwent surgical resection with clear margins by tumor; Denominator = number of patients underwent surgical resection). Standard: >90%; and (III) proper lymphadenectomy is curative and fundamental to the staging and defnition of prognosis (Numerator = number of patients with colon cancer operated with number of lymph nodes taken ≥12; Denominator = number of patients with colon cancer underwent surgical resection). Standard: ≥75%.
The main indicators for the pathological phase in the study are summarized below: (I) all patients underwent surgical resection of colon cancer, there should be a pathological report with a minimal set of data (Numerator = number of patients with colon cancer operated with evidence of the main parameters required; Denominator = number of patients underwent surgery for colon cancer). Standard: ≥95%; and (II) in all cases with colic tumor should be assigned a stage according to the TNM categories (Numerator = number of patients with colon cancer referredto our Clinical Oncology Unit and assigned a TNM stage, Denominator = number of patients with colon cancer referred to our Clinical Oncology Unit). Standard: ≥95%.
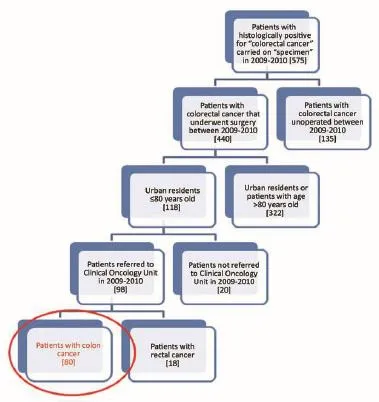
Figure 1Selection of study sample.
The main indicators for the oncological phase in the study are summarized below: (I) patients with stage III, taking into account the biological age and comorbidities, are candidates for adjuvant chemotherapy (Numerator = number of patients with colon cancer stage III operated with adjuvant chemotherapy, Denominator = total number of patients with cancer operated with stage III colon cancer). Standard: >90%; (II) adjuvant chemotherapy should be started within 6-8 weeks after surgery (Numerator = number of patients with colon cancer stage III operated with adjuvant chemotherapy who started chemotherapy within 6-8 weeks after surgery, Denominator=number of patients with colorectal cancer operated with stage III). Standard:>90%; (III) the optimal duration of adjuvant chemotherapy is 6 months (Numerator = number of patients with stage III colon cancer operated receiving adjuvant chemotherapy for 6 months, Denominator = number of patients with stage III colon cancer surgery). Standard: >90%; (IV) all patients underwent surgery for colon cancer should be included in a program of follow-up (Numerator = number of patients underwent surgery for colon cancer referred to our Clinical Oncology Unit in the 12 months following surgery, Denominator = number of patients underwent surgery for colon cancer). Standard: >90%; (V) it is recommended regular routine of the CEA (Numerator = number of patients operated for colon cancer referred to our Clinical Oncology Unit underwent CEA/year, Denominator = number of patients underwent surgery for colon cancer). Standard: >90%; (VI) it is recommended that regular routine liver ultrasound or CT scan in all patients who are candidates for hepatic resection considering the possible detection of liver lesions secondary operable (Numerator = number of patients underwent surgery for colon cancer have an ultrasound or CT scan for 12 months after liver surgery, Denominator = number of patients underwent surgery for colon cancer). Standard: >90%; and (VII) in patients in whom endoscopy showing “colon unharmed”repeat endoscopic examination is recommended after 1 year after surgery, then after 3 years and then every 5 years (Numerator = number of patients with colon cancer underwent endoscopic examination work within 1 year after surgery, Denominator = number of patients underwent surgery for colon cancer). Standard: >90%.
Results
Of the 575 patients analyzed as previously described and according to the criteria of inclusion and exclusion already mentioned, 80 were included in our analysis (Figure 1). We have considered two different populations: patients from the Regional Colorectal Cancer Screening Program (for which it is institutionally active the PDTA) and patients are not coming from that Regional Colorectal Cancer Screening Program.
Considering patients from the Regional Colorectal Cancer Screening Program (19 patients, corresponding to 24.0% of the general case study), 3 (15.8%) were deceased and 16 (84.2%) were alive without evidence of the disease (NED). Median age was 62 years (range, 50-72 years), 8 patients (42.1%) were male and 11 (57.9%) were female. Dukes stadia were: A in 8 patients (42.0%), B in 3 patients (15.8%), C in 4 patients (21.1%) and D in 4 patients (21.1%). All the results concerning the diagnostic, surgical, pathological and oncological phases were summarized in Figures 2-6.
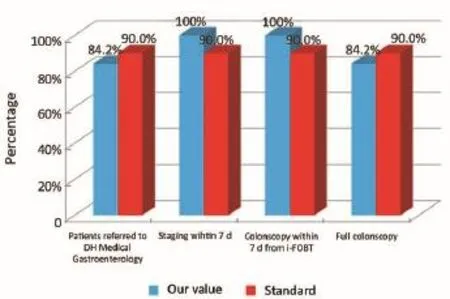
Figure 2Diagnostic phase (patients from the Regional Colorectal Cancer Screening Program).
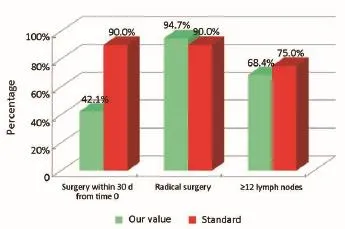
Figure 3Surgical phase (patients from the Regional Colorectal Cancer Screening Program).
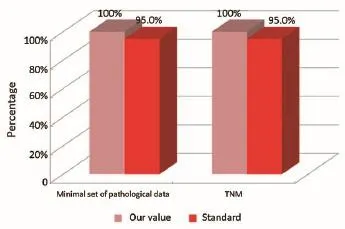
Figure 4Pathological phase (patients from the Regional Colorectal Cancer Screening Program).
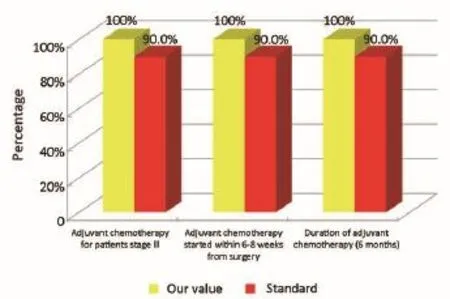
Figure 5Oncological phase (patients from the Regional Colorectal Cancer Screening Program).
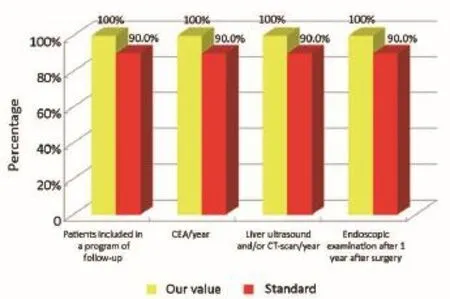
Figure 6Oncological phase (patients from the Regional Colorectal Cancer Screening Program).
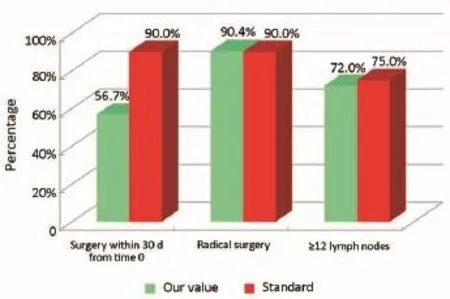
Figure 7Surgical phase (patients that are not coming from Regional Colorectal Cancer Screening Program).
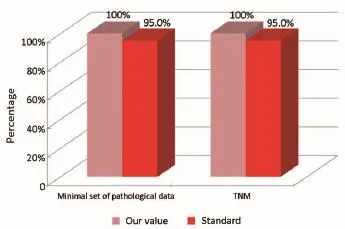
Figure 8Pathological phase (patients that are not coming from Regional Colorectal Cancer Screening Program).
Concerning patients that are not coming from Regional Colorectal Cancer Screening Program (61 patients, corresponding to 76.0% of the general case study), 9 patients (14.8%) were deceased, 43 patients (70.5%) were NED, 8 patients (13.1%) were alive with metastases and 1 patient (1.6%) was lost during follow-up (PFU). Median age was 70 years (range, 40-80 years), 24 patients (39.3%) were male and 37 patients (60.7%) were female. Dukes stadia was: A in 8 patients (13.1%), B in 24 patients (29.3%), C in 15 patients (24.6%) and D in 14 patients (23.0%). About this group of patients has not been possible to calculate the diagnostic phase, because the criteria referring to this specifc phase were only applicable to patients from the Regional Colorectal Cancer Screening Program. All the results concerning surgical, pathological and oncological phases were summarized in Figures 7-10.
Discussion
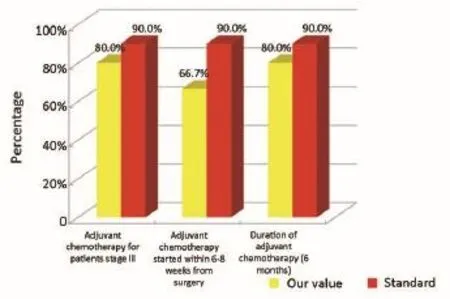
Figure 9Oncological phase (patients that are not coming from Regional Colorectal Cancer Screening Program).
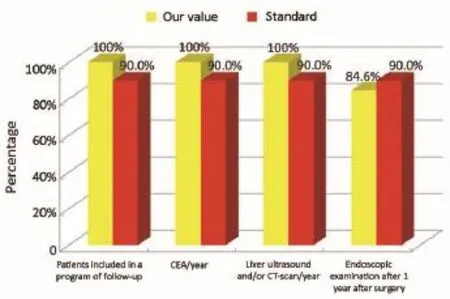
Figure 10Oncological phase (patients that are not coming from Regional Colorectal Cancer Screening Program).
Our experience has highlighted certain aspects, such as substantial compliance and/or proximity of the values obtained with the optimal standard of reference, keeping in mind the displacement relative to certain stages of the path and the assessment of their appropriateness. Concerning the diagnostic phase, we can see that there was a substantial compliance standard, with values very close to the optimum values to achieve. Regarding the surgical phase, data from both populations are essentially superimposable. A first critical detected is represented by the data of the value of the indicator relative to the time relapsed between the time 0 (date of endoscopy) and surgery, that was significantly lower compared to the standard required. Referring instead to the criteria for surgery, we can see that one side has been achieved clean margins of resection than the standard in all other groups, and a proportion of patients with a number of lymph nodes greater than or equal to 12, very close to the standard for all groups. Concerning the pathological phase, is to emphasize the fact of how the standard has been widely observed, with the presence in each reportof all the parameters mentioned above. It’s also unlikely as everything is simplified by the presence of a checklist summary of these parameters, besides the role it plays in facilitating the reading of the pathological report by the medical oncologist. Regarding the oncological phase, the analysis of parameters related to adjuvant chemotherapy in stage III showed that there was a substantial compliance with the optimal standards in all groups examined and when it apparently seems not to have happened, it can easily be explained by clinical decisions (e.g., comorbidity) or chosen by the patient (e.g., refusal to accept the adjuvant chemotherapy). The other aspect concerns the analysis of follow-up program, in which each patient is inserted, with a true respect of the timing and proximity to a nearly complete, especially as regards the performance of colonoscopies a year after surgery, the optimal standard.
The present experience has highlighted certain aspects, such as substantial compliance and/or proximity of the values obtained with the optimal standard of reference for all population groups, keeping in mind the displacement relative to certain phases of the roadmap and to assess their appropriateness. There are critical points such as surgical times and the proportion of patients who started chemotherapy within 6-8 weeks after surgery. As regards the first aspect there is to emphasize the fact of how the reading of the data can be misleading: it is true that the data obtained regarding the timing of surgery exceeds a few days, the default value as optimal standard, to where there is to add the observation that it is a ratio and then this discrepancy would be even more enhanced when viewed in terms of percentage. No delay seems rather to be due to having done or not pre-operative staging c/o DH Medical Gastroenterology. As for the second critical this may be partly due to possible delays related to the staging of disease and in part to the waiting time required for movement of the positioning of the central venous catheter (CVC) and to that extent the possible increase in the coming years of schemes that does not require the need to plant CVC (e.g., XELOX) could lead to a partial solution of the problem (7-9). And as normal, however, each value should always be regarded as subject to improvement. In the interests of this type, therefore, the presence of organized screening programs would ensure a better respect of the indicators for both the diagnostic stages that follow-up, impinging consequently also positively on the management of patients not incorporated in the screening.
From the data of national and international literature (2,3,10-14) we also desirable extension of PDTA (by taking charge of the patient at follow-up) not only to patients from the Regional Colorectal Cancer Screening Program, but at all patients with colon cancer, also in relation to data collected do not show a clear discrepancy in the quality of service offered to each other; from this point of view the creation of multidisciplinary clinics would ensure coordination between the various professionals and planning the best therapeutic strategy to follow. Finally we fnd it useful to encourage the spread of the culture of clinical audit particularly in areas in which multidisciplinary care is the cornerstone of a good quality of care (15-22). In the interests of this type, the creation of PDTA in various diseases with the detection of indicators of process and result (always referring to the Evidence-based Medicine) for the monitoring of the path and a periodic activity of clinical audit (as a means to continually and systematically monitor various indicators of quality education) may become an important tool to ensure and maintain quality care, in view of “continuous quality improvement”.
Acknowledgements
Disclosure: The authors declare no confict of interest.
1. Brenner H, Bouvier AM, Foschi R, et al. Progress in colorectal cancer survival in Europe from the late 1980s to the early 21st century: the EUROCARE study. Int J Cancer 2012;131:1649-58.
2. Zorzi M, Fedato C, Naldoni C, et al. Screening for colorectal cancer in Italy: 2007 survey. Epidemiol Prev 2009;33:57-74.
3. Matarese VG, Feo CV, Lanza G, et al. The frst 2 years of colorectal cancer screening in Ferrara, Italy. Eur J Cancer Prev 2011;20:166-8.
4. Scally G, Donaldson LJ. The NHS's 50 anniversary. Clinical governance and the drive for quality improvement in the new NHS in England. BMJ 1998;317:61-5.
5. Labianca R, Nordlinger B, Beretta GD, et al. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v70-7.
6. Van Cutsem E, Nordlinger B, Cervantes A, et al. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 2010;21 Suppl 5:v93-7.
7. André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin,fuorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51.
8. Glen H, Cassidy J. Redefning adjuvant chemotherapy in patients with stage III colon cancer: X-ACT trial. Expert Rev Anticancer Ther 2008;8:547-51.
9. Sargent D, Shi Q, Yothers G, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fuoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 2011;47:990-6.
10. Pezzoli A, Matarese V, Rubini M, et al. Colorectal cancer screening: results of a 5-year program in asymptomatic subjects at increased risk. Dig Liver Dis 2007;39:33-9.
11. Masseria C. Colorectal cancer in Italy: a review of current national and regional practice on screening and treatment. Eur J Health Econ 2010;10 Suppl 1:S41-9.
12. Labianca R, Merelli B. Screening and diagnosis for colorectal cancer: present and future. Tumori 2010;96:889-901.
13. Zorzi M, Baracco S, Fedato C, et al. Screening for colorectal cancer in Italy: 2008 survey. Epidemiol Prev 2010;34:53-72.
14. Senore C, Armaroli P, Silvani M, et al. Comparing different strategies for colorectal cancer screening in Italy: predictors of patients' participation. Am J Gastroenterol 2010;105:188-98.
15. Lembcke PA. Evolution of the medical audit. JAMA 1967;199:543-50.
16. Benjamin A. Audit: how to do it in practice. BMJ 2008;336:1241-5.
17. Smith R. Audit and research. BMJ 1992;305:905-6.
18. Hearnshaw HM, Baker RH, Robertson N. Multidisciplinary audit in primary healthcare teams: facilitation by audit support staff. Qual Health Care 1994;3:164-8.
19. Kitson A. Quality improvement: a multiprofessional commodity? Qual Health Care 1996;5:65-6.
20. Hysong SJ. Meta-analysis: audit and feedback features impact effectiveness on care quality. Med Care 2009;47:356-63.
21. Whittle C, Hewison A. Integrated care pathways: pathways to change in health care? J Health Organ Manag 2007;21:297-306.
22. Degeling PJ, Maxwell S, Iedema R, et al. Making clinical governance work. BMJ 2004;329:679-81.
Cite this article as:Giuliani J, Marzola M. A path for diagnosis and therapy of colon cancer: a continuous quality improvement. Chin J Cancer Res 2014;26(6):717-723. doi: 10.3978/ j.issn.1000-9604.2014.12.08
10.3978/j.issn.1000-9604.2014.12.08
Submitted May 29, 2014. Accepted for publication Nov 25, 2014.
 Chinese Journal of Cancer Research2014年6期
Chinese Journal of Cancer Research2014年6期
- Chinese Journal of Cancer Research的其它文章
- Comparison of three mathematical prediction models in patients with a solitary pulmonary nodule
- Application of CUSA Excel ultrasonic aspiration system in resection of skull base meningiomas
- Knockdown of HMGB1 improves apoptosis and suppresses proliferation and invasion of glioma cells
- Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance
- Impact of jejunostomy during esophagectomy for cancer on health related quality of life
- Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2-T4 N0M0 and metastasis predictive equation
