Impact of jejunostomy during esophagectomy for cancer on health related quality of life
Surgical Oncology Unit, Veneto Institute of Oncology (IOV-IRCCS), Padua 35128, Italy
*These two authors contributed equally to this work.
Correspondence to: Marco Scarpa, MD. Surgical Oncology Unit, Veneto Oncology Institute (IOV-IRCCS), Via Gattamelata 64, Padua 35128, Italy. Email: marcoscarpa73@yahoo.it.
Impact of jejunostomy during esophagectomy for cancer on health related quality of life
Marco Scarpa*, Francesco Cavallin*, Giulia Noaro, Eleonora Pinto, Rita Alferi, Matteo Cagol, Carlo Castoro
Surgical Oncology Unit, Veneto Institute of Oncology (IOV-IRCCS), Padua 35128, Italy
*These two authors contributed equally to this work.
Correspondence to: Marco Scarpa, MD. Surgical Oncology Unit, Veneto Oncology Institute (IOV-IRCCS), Via Gattamelata 64, Padua 35128, Italy. Email: marcoscarpa73@yahoo.it.
Background:The aim of this study was to evaluate the impact of jejunostomy during esophagectomy for cancer on postoperative health-related quality of life (HRQL).
Methods:We evaluate all consecutive patients who underwent esophagectomy for cancer at the surgical oncology unit of the Veneto Institute of Oncology (IOV-IRCCS) between January 2008 and March 2014. The primary outcome was HRQL, which was assessed using nine scales of EORTC C30 and OES18 questionnaires. General linear models were estimated to evaluate mean score difference (MD) of each selected scale in patients with and without jejunostomy, adjusting for clinically relevant confounders. The secondary outcomes were morbidity, hospital stay, postoperative weight loss and postoperative albumin impairment.
Results:Jejunostomy was performed in 40 on 109 patients (41.3%) who participated in quality of life investigation. A clinically and statistically significantly worse eating at admission (P=0.009) became not clinically significant at 3 months after surgery (MD =9.1). Jejunostomy was associated to clinically and statistically signifcantly poorer emotional function (EF) at 3 months after surgery (MD =−15.6; P=0.04). Hospital stay was longer in jejunostomy group (median, 20 vs. 17 days, P=0.02).
Conclusions:In our series patients who had a jejunostomy during esophagectomy had been selected for their risk for postoperative complication. However, their postoperative outcome was actually similar compared to those without jejunostomy. Nevertheless, jejunostomy was associated to clinically and statistically signifcantly poorer EF at 3 months after surgery. Therefore, patient candidate to esophagectomy and feeding jejunostomy should receive additional psychological support.
Health-related quality of life (HRQL); jejunostomy; esophagectomy; esophageal cancer
View this article at:http://dx.doi.org/10.3978/j.issn.1000-9604.2014.12.16
Introduction
Esophageal cancer incidence is steadily increasing in Western world (1) but its prognosis remains poor. Combined modality treatment protocols such as neoadjuvant radiation and/or chemotherapy followed by surgery is the current treatment option and meta-analyses of randomized trials have found some survival advantages (2,3), although only patients with a complete pathologic response to neoadjuvant therapy seem to enjoy a signifcantly better chance of survival (4,5). However, esophagectomy remains the standard treatment for patients presenting with resectable esophageal cancer but it is associated with a high risk of serious complications (6-11).
Alongside postsurgical survival, morbidity and mortality rates (6), health-related quality of life (HRQL) has recently become an important outcome parameter to evaluate esophagectomy for esophageal cancer (12). Risk factors for poor postoperative HRQL are now, in fact, evaluated carefully when clinical decisions are being made and patients are informed about long-term consequences of surgery. In a systematic review global HRQL and physicalfunction resulted signifcantly worsened during the 6-month following esophagectomy (13).
In our previous investigation on quality of life after esophagectomy (14,15) we had observed, but not reported, an unexplained and unexpected negative effect of jejunostomy placement during esophagectomy on postoperative quality of life. In a recent meta-analysis including 3,293 patients, jejunostomy tube feeding was effective in meeting short-term nutritional requirements after esophagectomy, but major tube-related complications necessitated relaparotomy in 0-2.9% of patients (16). However, the authors pointed out that data on patient satisfaction and quality of life were not found for any of the feeding routes investigated (16).
Therefore, the aim of this study was to evaluate the impact of jejunostomy during esophagectomy for cancer on postoperative HRQL.
Methods
Study design
All consecutive patients who underwent esophagectomy for cancer at the surgical oncology unit of the Veneto Institute of Oncology (IOV-IRCCS) between January 2008 and March 2014 were retrospectively evaluated for inclusion in the study. Information on quality of life was available in 109 patients who accepted to fill the questionnaires and they were included in the study analysis. Patients who received jejunostomy were compared with those who did not received jejunostomy. The primary outcome was HRQL. The secondary outcomes were morbidity, hospital stay, postoperative weight loss (at 3 months after surgery) and postoperative albumin impairment (at 2nd-3rdpostoperative week). The study was conducted according to Helsinki Declaration principles and patients gave their consent to have their data collected for scientific purpose. This retrospective study was notifed to the Ethical Committee of IOV-IRCCS.
Health-related quality of life (HRQL)
HRQL was assessed using the EORTC QLQ-C30 and OES-18 questionnaires (17,18) at admission and at 3 months after surgery. Specific aspects were selected a priori, whereas the remaining aspects were not analyzed. The selected aspects were global quality of life (C30 QL2), all C30 functional scales [C30 physical function (PF2), role function (RF2), social function (SF), emotional function (EF), cognitive function (CF)], fatigue (C30 FA), dysphagia (OES18 DYS) and eating (OES18 EAT). All scores range from 0 to 100, with a high score representing healthy status for functional scales and the global health status scale, but high level of symptomatology/problems for symptom scales (apart from dysphagia).
Treatment
Tumor staging was performed according to the criteria of the International Union Against Cancer (19). Patients with tumor staged above T3N0 or anyTN1 were offered neoadjuvant therapy as described elsewhere (14). Patients were considered resectable when staged below T3N0 or, after the termination of neoadjuvant treatment, when there was no evidence of distant metastases or locally advanced tumor with gross periesophageal involvement at restaging.
Surgical technique
Details concerning surgical techniques have been published elsewhere (20). Briefly, esophagectomy was performed using an Ivor-Lewis procedure, via a laparotomy and right thoracotomy, for tumors of the mid-lower esophagus and gastric cardia. A three-stage McKeown’s procedure, with an additional left cervical incision, was reserved for tumors in the upper third of the esophagus. At least 6-8 cm of healthy esophagus was resected above the proximal edge of the tumor to avoid neoplastic involvement of the resection margins. In this group of patients en bloc lymph node dissection was performed, including the paraesophageal, sub carinal, posterior mediastinal and paracardial lymph nodes, as well as those located along the lesser gastric curvature, the origin of the left gastric artery, the celiac trunk, the common hepatic artery and the splenic artery.
Jejunostomy was performed using a standard approach before abdominal wall closure. The first jejunal loop beyond the ligament of Treitz was selected and a feeding tube (10 Fr duodenal tube; length, 125 cm) was passed through the abdominal wall, advanced 4 cm in a submucosal tunnel, and fed through the mucosa into the jejunal lumen. The catheter was advanced 20 cm and secured with an absorbable purse string suture. An additional seromuscular Witzel (21) tunnel was fashioned to overlap the catheter and the purse-string suture site. Subsequently, the jejunum was secured to the anterior abdominal wall with interrupted absorbable sutures. Nasogastric (NG) tubes were placed in allpatients until the radiological assessment of the anastomosis.

Table 1Patients’ characteristics
Statistical analysis
Continuous data are expressed as median and interquartile range (IQR). Categorical data were compared between the two groups using Fisher’s test and continuous data using Mann-Whitney test. The selected aspects of EORTC questionnaires were calculated according to the developers’ instructions and were reported as mean score differences (MDs) between patients with and without jejunostomy. A MD of ten points or more was considered clinically relevant (22) and any such difference was tested for statistical significance. A general linear model was estimated to evaluate the effect of jejunostomy on clinically relevant MD at admission adjusting for age, tumor site and pathologic stage. A general linear model was estimated to evaluate the effect of jejunostomy on clinically relevant MD at 3 months after surgery adjusting for age, tumor site, pathologic stage and morbidity. Statistical analysis was performed using SAS 9.1 and a P value less than 0.05 was considered signifcant.
Results
Sample characteristics
Among the group of patients who accepted to fill the questionnaires and thus were included in the study, a feeding jejunostomy was performed during esophagectomy in 40 on 109 patients (36.7%) and all jejunostomies were removed between 2 and 3 months. No jejunostomy related complication was observed in the study group. Patients with jejunostomy were older than those without jejunostomy (median, 64 vs. 59 years, P=0.02). In addition, jejunostomy was performed mostly in patients with SCC hystotype (P=0.0001) and upper tumor sites (P<0.0001). Patients’characteristics are shown in Table 1.
Health-related quality of life (HRQL)
At admission, patients with jejunostomy had similar functional scales than those without jejunostomy (Table 2 and Figure 1), but they reported a clinically and statistically signifcantly poorer EF at 3 months after surgery (MD =−15.6; P=0.04). In addition, a clinically significantly poorer physical function was observed in patients with jejunostomy at 3 months after surgery (MD =−15.2; P=0.07).
Patients with jejunostomy reported a clinically and statistically significantly worse eating (EAT scale) at admission than those without jejunostomy (P=0.009; Table 2). However, this difference became not clinically significant at 3 months after surgery (MD =9.1; Table 2 and Figure 2). In addition, a clinically significantly worse dysphagia was observed in patients with jejunostomy at admission (MD =−12.2; P=0.16), but it became not clinically signifcant at 3 months after surgery (MD =−0.4; Table 2 and Figure 2).
Secondary outcomes
A similar morbidity rate was observed in patients withand without jejunostomy (35.0% vs. 21.7%, respectively; P=0.18). Hospital stay was longer in jejunostomy group (median, 20 vs. 17 days, P=0.02; Table 3), whereas postoperative weight loss and albumin levels were similar in the two groups (P=0.94 and P=0.31; Table 3). In addition, patients with jejunostomy experienced a similar (P=0.74) postoperative albumin impairment (median 12, IQR 9-15) than those without jejunostomy (median 12, IQR 8-15).
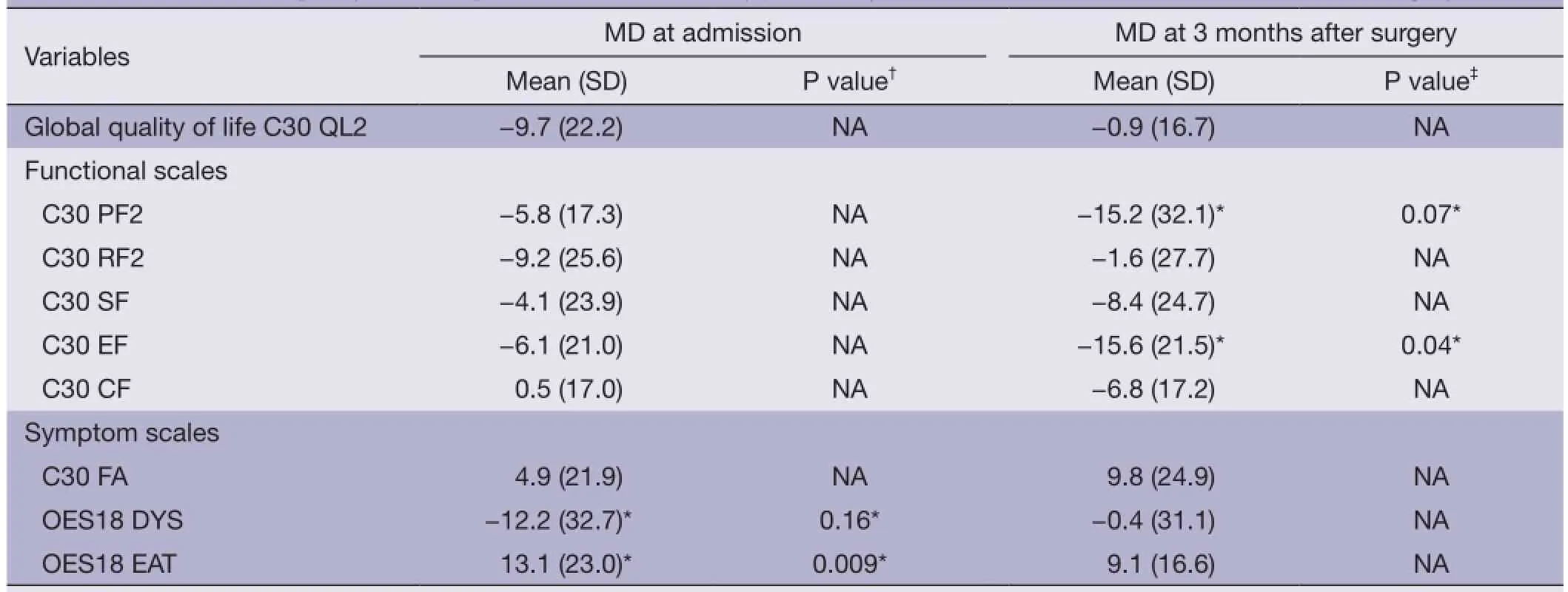
Table 2Health-related quality of life in patients with or without jejunostomy, assessed at admission and at 3 months after surgery
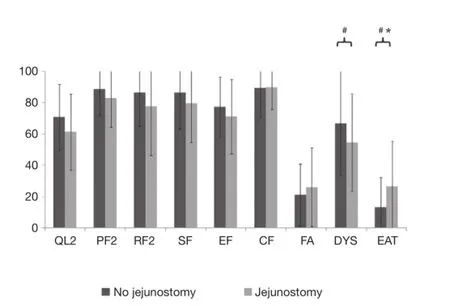
Figure 1HQRL mean values (with SD bars limited in the 0-100 interval) in patients with or without jejunostomy at admission for surgery.#, clinically signifcant (MD ≥10). *, statistically signifcant (P<0.05). QL2: C30 global quality of life; PF2: C30 physical function; RF2: C30 role function; SF: C30 social function; EF: C30 emotional function; CF: C30 cognitive function; FA: C30 fatigue; DYS: OES18 dysphagia; EAT: OES18 eating; MD, mean score difference.
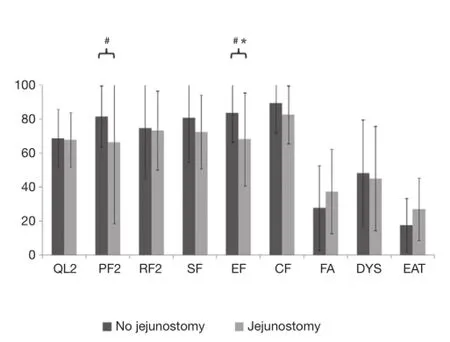
Figure 2HQRL mean values (with SD bars limited in the 0-100 interval) in patients with or without jejunostomy at 3 months after surgery.#, clinically signifcant (MD ≥10). *, statistically signifcant (P<0.05). QL2: C30 global quality of life; PF2: C30 physical function; RF2: C30 role function; SF: C30 social function; EF: C30 emotional function; CF: C30 cognitive function; FA: C30 fatigue; DYS: OES18 dysphagia; EAT: OES18 eating; MD, mean score difference.
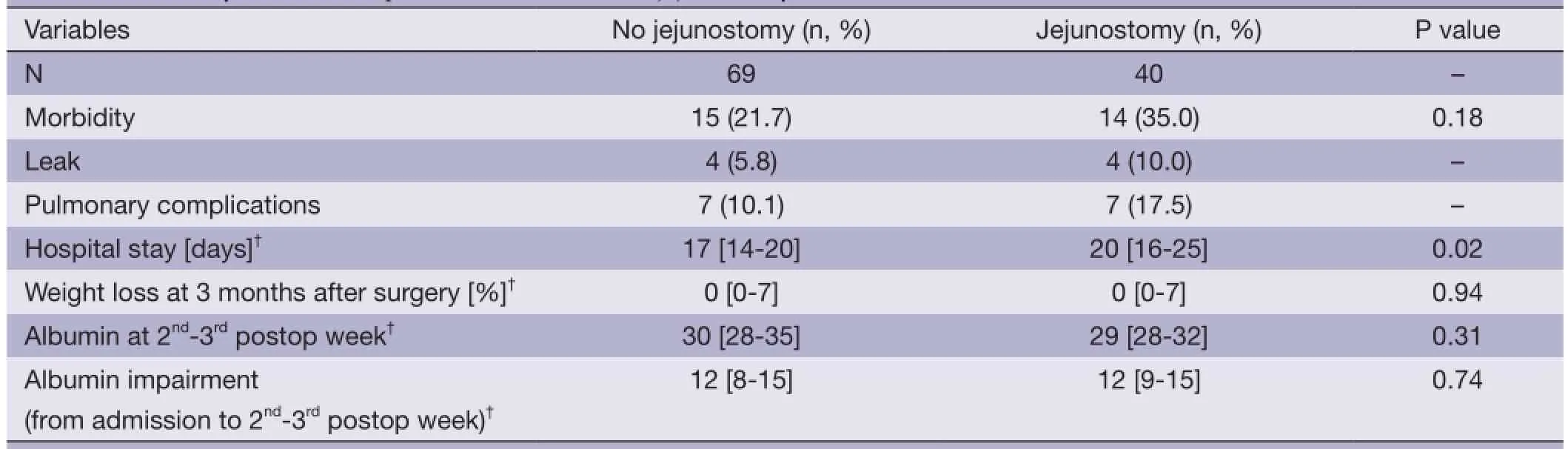
Table 3Secondary outcomes in patients with or without jejunostomy
Discussion
Esophagectomy remains the standard treatment for patients presenting with resectable esophageal cancer but it is associated with a high risk of serious complications and post-operative nutritional impairment (6-11). Few studies have assessed the potential benefits of continuing supplementary jejunostomy feeding after hospital discharge (23). In an institutional review of practice (24) comparing the historical practice of not providing home enteral support with the recent practice of home jejunostomy feeding, home enteral support was associated with better weight maintenance. Nevertheless, the effect of enteral nutrition on other outcome measures such as quality of life is not known. Therefore, the aim of this study was to evaluate the impact of jejunostomy during esophagectomy for cancer on postoperative HRQL.
In our series, patients who had a jejunostomy were significantly older than those without jejunostomy and their cancer was more often a squamous cell carcinoma and was more often located in the upper esophagus. These differences directly refected the selection of patients who were candidate to have a temporary jejunostomy beside esophagectomy. In fact, as pointed out by Srinathan et al. (25), it seems reasonable to adopt the practice of using feeding jejunostomy tubes in patients who the surgeon feels are at elevated risk for anastomotic failure, which will delay commencement of oral intake. Indeed, our patients who had jejunostomy clearly were more frail patients (i.e., patients with preoperative food intake impairment, patients who had upper esophageal cancer and elderly patients).
In our series, at admission, patients with jejunostomy had similar functional scales than those without jejunostomy, but they reported a clinically and statistically significantly poorer EF at three months after surgery. According to recent studies that prospectively analyzed quality of life after esophagectomy in British and Dutch patients, EF resulted signifcantly improved at the six month follow-up (26-28). Furthermore, Swedish patients reported significantly better EF even three years after esophagectomy (29). The worsening in EF observed in patients with a jejunostomy may be due to the subjective perception of still being ill caused by the feeding tube and the daily practice related to this unnatural way of feeding (30). Moreover, in patients with a jejunostomy the body image may be heavily impaired, thus affecting their EF (31). In our opinion, patients who need a jejunostomy because of substantial weight loss before surgery, advanced age or upper esophageal cancer, may possibly benefit of a dedicated psychological support to cope with this new body situation and to recover their EF.
In our series, in spite of a similar morbidity rate observed in patients with and without jejunostomy, hospital stay was longer in jejunostomy group. This data seem to be related to the general higher frailty of patients who had a jejunostomy, a longer time need to recover after a cervicalanastomosis and longer time need to organize home enteral feeding. On the other hand, postoperative weight loss, albumin levels and postoperative albumin impairment were similar in the two groups suggesting that jejunostomy, even if it did not completely corrected the physiological postoperative nutritional impairment, at least prevented further worsening in patients particularly at risk. In fact, in a previous study we had observed that patients after esophagectomy lose weight up to six month after the operation (32). Tube feeding provided a relief in those who might have lose much more weight.
Finally, a clinically signifcantly poorer physical function was observed in patients with jejunostomy at 3 months after surgery. In our previous study (13), we had observed that in patients who had postoperative complications physical function tended to be worse than in those who had an uneventful recovery. From patient’s point of view jejunostomy is a postoperative complication that limits his physical activity and thus recovery. Therefore, it seems reasonable to limit the practice of using feeding jejunostomy tubes in patients who the surgeon feels are at elevated risk for anastomotic failure, which will delay commencement of oral intake (25).
Conclusions
In conclusion, in our series patients who had a jejunostomy during esophagectomy had been selected for their risk for postoperative complication. However, their postoperative outcome was actually similar compared to those without jejunostomy. Nevertheless, jejunostomy was associated to clinically and statistically significantly poorer EF at 3 months after surgery. Therefore, patient candidate to esophagectomy and feeding jejunostomy should receive additional psychological support.
Acknowledgements
Funding: This study was supported by Current Research Fund from Italian Ministry of Health to Carlo Castoro and by a grant from Berlucchi Foundation (Brescia, Italy) to Carlo Castoro.
Disclosure: The authors declare no confict of interest.
1. Jemal A, Murray T, Samuels A, et al. Cancer statistics, 2003. CA Cancer J Clin 2003;53:5-26.
2. Gebski V, Burmeister B, Smithers BM, et al. Survival benefts from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226-34.
3. Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a metaanalysis. Surgery 2005;137:172-7.
4. Zacherl J, Sendler A, Stein HJ, et al. Current status of neoadjuvant therapy for adenocarcinoma of the distal esophagus. World J Surg 2003;27:1067-74.
5. Brücher BL, Stein HJ, Zimmermann F, et al. Responders beneft from neoadjuvant radiochemotherapy in esophageal squamous cell carcinoma: results of a prospective phase-II trial. Eur J Surg Oncol 2004;30:963-71.
6. Ruol A, Castoro C, Portale G, et al. Trends in management and prognosis for esophageal cancer surgery: twenty-fve years of experience at a single institution. Arch Surg 2009;144:247-54; discussion 254.
7. De Vita F, Di Martino N, Orditura M, et al. Preoperative chemoradiotherapy for squamous cell carcinoma and adenocarcinoma of the esophagus: a phase II study. Chest 2002;122:1302-8.
8. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52.
9. Wu PC, Posner MC. The role of surgery in the management of oesophageal cancer. Lancet Oncol 2003;4:481-8.
10. Jamieson GG, Mathew G, Ludemann R, et al. Postoperative mortality following oesophagectomy and problems in reporting its rate. Br J Surg 2004;91:943-7.
11. Viklund P, Lindblad M, Lu M, et al. Risk factors for complications after esophageal cancer resection: a prospective population-based study in Sweden. Ann Surg 2006;243:204-11.
12. McLeod RS. Quality-of-life measurement in the assessment of surgical outcome. Adv Surg 1999;33:293-309.
13. Scarpa M, Valente S, Alferi R, et al. Systematic review of health-related quality of life after esophagectomy for esophageal cancer. World J Gastroenterol 2011;17:4660-74.
14. Scarpa M, Saadeh LM, Fasolo A, et al. Health-related quality of life in patients with oesophageal cancer: analysis at different steps of the treatment pathway. J Gastrointest Surg 2013;17:421-33.
15. Scarpa M, Pinto E, Saadeh LM, et al. Sleep disturbances and quality of life in postoperative management after esophagectomy for esophageal cancer. World J Surg Oncol 2014;12:156.
16. Weijs TJ, Berkelmans GH, Nieuwenhuijzen GA, et al.Routes for early enteral nutrition after esophagectomy. A systematic review. Clin Nutr 2014. [Epub ahead of print].
17. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76.
18. Blazeby JM, Conroy T, Hammerlid E, et al. Clinical and psychometric validation of an EORTC questionnaire module, the EORTC QLQ-OES18, to assess quality of life in patients with oesophageal cancer. Eur J Cancer 2003;39:1384-94.
19. Edge SB, Byrd DR, Compton CC, et al. eds. AJCC Cancer Staging Manual (ed 7). New York: Springer-Verlag, 2009:117-26.
20. Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little infuence on outcome and survival. J Thorac Cardiovasc Surg 2007;133:1186-92.
21. Witzel O. Zur technik der magenfstelanlegung. Zentralbl Chir 1891;18:601-4.
22. Osoba D, Rodrigues G, Myles J, et al. Interpreting the signifcance of changes in health-related quality-of-life scores. J Clin Oncol 1998;16:139-44.
23. Hyltander A, Bosaeus I, Svedlund J, et al. Supportive Nutrition on Recovery of Metabolism, Nutritional State, Health-related Quality of Life, and Exercise Capacity After Major Surgery: A Randomised Study. Clin Gastroenterol Hepatol 2005;3:466-74.
24. Macharg FM, Huddy JR, Priest OH, et al. Extended enteral nutrition via feeding jejunostomy reduces weight loss and readmission following esophagectomy. Dis Esophagus 2012;25:48A.
25. Srinathan SK, Hamin T, Walter S, et al. Jejunostomy tube feeding in patients undergoing esophagectomy. Can J Surg 2013;56:409-14.
26. Reynolds JV, McLaughlin R, Moore J, et al. Prospective evaluation of quality of life in patients with localized oesophageal cancer treated by multimodality therapy or surgery alone. Br J Surg 2006;93:1084-90.
27. Avery KN, Metcalfe C, Barham CP, et al. Quality of life during potentially curative treatment for locally advanced oesophageal cancer. Br J Surg 2007;94:1369-76.
28. van Meerten E, van der Gaast A, Looman CW, et al. Quality of life during neoadjuvant treatment and after surgery for resectable esophageal carcinoma. Int J Radiat Oncol Biol Phys 2008;71:160-6.
29. Viklund P, Lindblad M, Lagergren J. Infuence of surgeryrelated factors on quality of life after esophageal or cardia cancer resection. World J Surg 2005;29:841-8.
30. Scarpa M, Angriman I, Ruffolo C, et al. Healthrelated quality of life after restorative proctocolectomy for ulcerative colitis: long-term results. World J Surg 2004;28:124-9.
31. Scarpa M, Erroi F, Ruffolo C, et al. Minimally invasive surgery for colorectal cancer: quality of life, body image, cosmesis, and functional results. Surg Endosc 2009;23:577-82.
32. Scarpa M, Cagol M, Bettini S, et al. Overweight patients operated on for cancer of the esophagus survive longer than normal-weight patients. J Gastrointest Surg 2013;17:218-27.
Cite this article as:Scarpa M, Cavallin F, Noaro G, Pinto E, Alfieri R, Cagol M, Castoro C. Impact of jejunostomy during esophagectomy for cancer on health related quality of life. Chin J Cancer Res 2014;26(6):678-684. doi: 10.3978/ j.issn.1000-9604.2014.12.16
10.3978/j.issn.1000-9604.2014.12.16
Submitted Nov 29, 2014. Accepted for publication Dec 05, 2014.
 Chinese Journal of Cancer Research2014年6期
Chinese Journal of Cancer Research2014年6期
- Chinese Journal of Cancer Research的其它文章
- Endobronchial ultrasound-guided transbronchial needle aspiration: unraveling myths of mass in the chest
- Next generation sequencing, inter-tumor heterogeneity and prognosis of hepatitis B related hepatocellular carcinoma
- Endoscopic ultrasonography: an advancing option with duality in both diagnosis and treatment of gastrointestinal oncology
- Use of endoscopic ultrasound-based techniques in tumor of the guts and beyond
- Quantitative index calculated by99mTc-GSA scintigraphy
- New ‘multi-omics’ approach and its contribution to hepatocellular carcinoma in China
