Efficacy of third-line pemetrexed monotherapy versus pemetrexed combination with bevacizumab in patients with advanced EGFR mutation-positive lung adenocarcinoma
National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, Department of Medicine, Guangzhou Institute of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou 510120, China
*These authors contributed equally to this work.
Correspondence to: Shi-Yue Li, MD; Rong-Chang Chen, MD. National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, Department of Medicine, Guangzhou Institute of Respiratory Disease, The First Affliated Hospital of Guangzhou Medical University, Guangzhou Medical University, 151 Yanjiang Road, Guangzhou 510120, China. Email: doctorzcz@163.com; chenrc@vip.163.com.
Efficacy of third-line pemetrexed monotherapy versus pemetrexed combination with bevacizumab in patients with advanced EGFR mutation-positive lung adenocarcinoma
Cheng-Zhi Zhou*, Yin-Yin Qin*, Zhan-Hong Xie, Jie-Xia Zhang, Ming Ou-Yang, Shi-Yue Li, Rong-Chang Chen
National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, Department of Medicine, Guangzhou Institute of Respiratory Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangzhou 510120, China
*These authors contributed equally to this work.
Correspondence to: Shi-Yue Li, MD; Rong-Chang Chen, MD. National Clinical Research Center for Respiratory Disease, State Key Laboratory of Respiratory Disease, Department of Medicine, Guangzhou Institute of Respiratory Disease, The First Affliated Hospital of Guangzhou Medical University, Guangzhou Medical University, 151 Yanjiang Road, Guangzhou 510120, China. Email: doctorzcz@163.com; chenrc@vip.163.com.
Objective:The purposes of this study were to observe the effects of different treatment strategies, including third-line pemetrexed alone versus its combination with bevacizumab, in patients with advanced epidermal growth factor receptor (EGFR) mutation-positive lung adenocarcinoma, and to analyze the effects of the different medication orders of frst- and second-line drugs on third-line effcacy.
Patients and methods:One hundred and sixteen cases of patients with EGFR-positive lung adenocarcinoma who had received third-line pemetrexed alone or in combination with bevacizumab between March 2010 and March 2014 at Guangzhou Institute of Respiratory Diseases, the First Affliated Hospital of Guangzhou Medical University were analyzed retrospectively. Additionally, all the patients were treated with frst-line gemcitabine and cisplatin (GP) chemotherapy and second-line EGFR tyrosine kinase inhibitor (TKI) or with frst-line EGFR-TKI and second-line GP chemotherapy.
Results:The median survival of 61 cases with third-line pemetrexed monotherapy was 36.22 months, the median survival time of 55 cases with third-line pemetrexed plus bevacizumab was 38.76 months, and there was a signifcant difference in survival time between the two groups (P=0.04). Subgroup analysis revealed that among the 55 cases with third-line bevacizumab plus pemetrexed treatment, the median survival of 29 patients with frst-line GP and second-line EGFR-TKI was 42.80 months, while the median survival of 26 patients with frst-line EGFR-TKI and second-line GP was only 34.46 months; additionally, there was a signifcant difference in the survival time between the two subgroups (P=0.001). Among 61 cases with thirdline pemetrexed treatment, the median survival of 34 patients with frst-line GP and second-line EGFR-TKI was 38.72 months, while the median survival of 27 patients with frst-line EGFR-TKI and second-line GP was only 32.94 months; the survival time of the two subgroups was signifcantly different (P=0.001).
Conclusions:Regardless of the order of the first- and second-line chemotherapy and TKI therapy, the pemetrexed plus bevacizumab regimen was superior to the pemetrexed monotherapy as the third-line therapy in patients with advanced EGFR-positive lung adenocarcinoma. However, this strategy is worth further investigation in prospective studies.
Epidermal growth factor receptor (EGFR) mutation; lung adenocarcinoma; pemetrexed; bevacizumab
View this article at:http://dx.doi.org/10.3978/j.issn.1000-9604.2014.12.19
Introduction
Lung adenocarcinoma is the main type of lung cancer (1,2), and several prospective clinical studies have confirmed that epidermal growth factor receptor (EGFR) mutationsensitive lung adenocarcinoma patients may be the targeted population of EGFR tyrosine kinase inhibitors (TKIs), after EGFR-TKI therapy, the progression-free survival (PFS) and overall survival (OS) of these patients were significantly longer than those with chemotherapy alone (3-5). Different from conventional cancer chemotherapy patterns, EGFR-TKI targeted therapy inhibits tumor cell proliferation by suppressing specific cell signaling transduction related to the occurrence and development of the tumor (6,7). Significant clinical efficacy has been achieved, as a survival benefit was noted for patients with EGFR mutation-sensitive lung cancer in the frst-, secondor third-line therapy (6). Compared with conventional cytotoxic chemotherapy, EGFR-TKI targeted therapy has better selectivity and shows relatively minor damage to normal cells (8-11). However, drug resistance appears in almost all patients after a certain period of time. The PFS of most EGFR mutation-positive patients fails to exceed 12 to 14 months (6). How to choose third-line treatment after receiving two lines of treatment, or whether the different medication orders of frst- and second-line in the first two lines of therapy affect the efficacy of the thirdline treatment, has not yet been reported. Due to various factors, bevacizumab and pemetrexed in particular are currently classified as self-financed drug items in most areas of China. Thus, many patients in China received first-line cisplatin-based chemotherapy and second-line EGFR-TKI therapy or first-line EGFR-TKI and secondline of cisplatin-based chemotherapy, but didn’t receive pemetrexed or bevacizumab. Considering this phenomenon, a retrospective analysis was conducted on selected cases of EGFR mutation-sensitive lung cancer patients treated with third-line pemetrexed monotherapy or cisplatinbased chemotherapy in combination with bevacizumab at Guangzhou Institute of Respiratory Diseases, the First Affiliated Hospital of Guangzhou Medical University between March 2010 and March 2014.
Patients and methods
Patients
One hundred and sixteen cases of patients with EGFR mutation-positive lung cancer, who had receivedgemcitabine and cisplatin (GP) chemotherapy, one type of EGFR-TKI therapy and a third-line application of pemetrexed alone or in combination with bevacizumab at Guangzhou Institute of Respiratory Diseases, the First Affiliated Hospital of Guangzhou Medical University between March 2010 and March 2014, were retrospectively reviewed. The diagnosis of all the patients was confirmed as EGFR mutation-sensitive positive adenocarcinoma by histopathology and cytology. Among them, there were 73 cases of EXON-19DEL, 37 cases of EXON-21-L858R and 6 cases of other rare mutations. The lesions were computed tomography (CT) measurable, heart, kidney and liver functions were normal, and no bone marrow suppression was found. All the patients received two-line therapy, 63 cases of first-line GP and second-line EGFRTKI, and 53 cases of first-line EGFR-TKI and secondline GP. The median age of all the patients was 59 years old (range, 38-79 years old) (Table 1).

Table 1Baseline of the two groups of patients

Table 2Effcacy of the third-line pemetrexed monotherapy group, pemetrexed plus bevacizumab group and subgroups
Treatment
In pemetrexed monotherapy group, pemetrexed 500 mg/m2was added to 100 mL of saline, and the solution was administered over 10 min of intravenous infusion every 21 days for one cycle. Daily oral supplements of 400 μg of folic acid were started 1 week before pemetrexed therapy, and an intramuscular injection of vitamin B12 1,000 μg was repeated every 9 weeks until 3 weeks after the end of the final treatment cycle. Dexamethasone tablets (4 mg) were orally administered 1 day before and on the frst and second day of pemetrexed treatment, twice a day. In pemetrexed plus bevacizumab combination group, bevacizumab 7.5 mg/kg, diluted with 100 mL of 0.9% sodium chloride, was administered by intravenous infusion on the frst day after chemotherapy (the frst infusion time >90 min, no less than 30~60 min afterwards); pemetrexed 500 mg/m2, dissolved in 100 mL of 0.9% sodium chloride solution, was administered intravenously by infusion on the second day (intravenous infusion time >10 min), and the pretreatment of pemetrexed was the same as the pemetrexed monotherapy group.
Ef fi cacy evaluation
Response Evaluation Criteria in Solid Tumors (RECIST) criteria were utilized to assess effcacy, including complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), the objective response rate [ORR; percentage of (CR + PR) patients of the entire group], and the disease control rate [DCR; percentage of (CR + PR + SD) patients of the entire group]. Third-line PFS refers to the time from the frst third-line treatment to PD in patients. OS refers to the time from the diagnosis to any cause of death. Subgroup survival curves are plotted with the time of observation on the horizontal axis and the survival rate on the vertical axis; each time point is connected to its corresponding survival rate.
Statistical analysis
Using Statistical Product and Service Solutions (SPSS) 16.0 software, effcacy analysis was performed using the t-test and self-control t-test. The Kaplan-Meier method was used for survival analysis. T wo-tailed tests were performed, and P values less than 0.05 were considered to be statistically signifcant.
Results
Ef fi cacy
After third-line treatment, the ORRs of patients in the pemetrexed monotherapy group and pemetrexed plus bevacizumab dual-drug combination group were 24.59% and 27.27%, respectively; and the DCRs were 77.05% and 80.00%, respectively. The difference was not statistically signifcant (Table 2).
In the pemetrexed plus bevacizumab dual-drug combination group of 55 cases, there were 29 cases of frstline GP and second-line EGFR-TKI and 26 cases of frstline EGFR-TKI and second-line GP. Subgroup analysis showed that the short-term efficacy of first-line GP and second-line EGFR-TKI in 29 patients (ORR and DCR were 31.03% and 82.76%, respectively) was better than that of frst-line EGFR-TKI and second-line GP in 26 patients (ORR and DCR were 23.08% and 76.92%, respectively); however, no statistical difference was reached (Table 2).
Survival
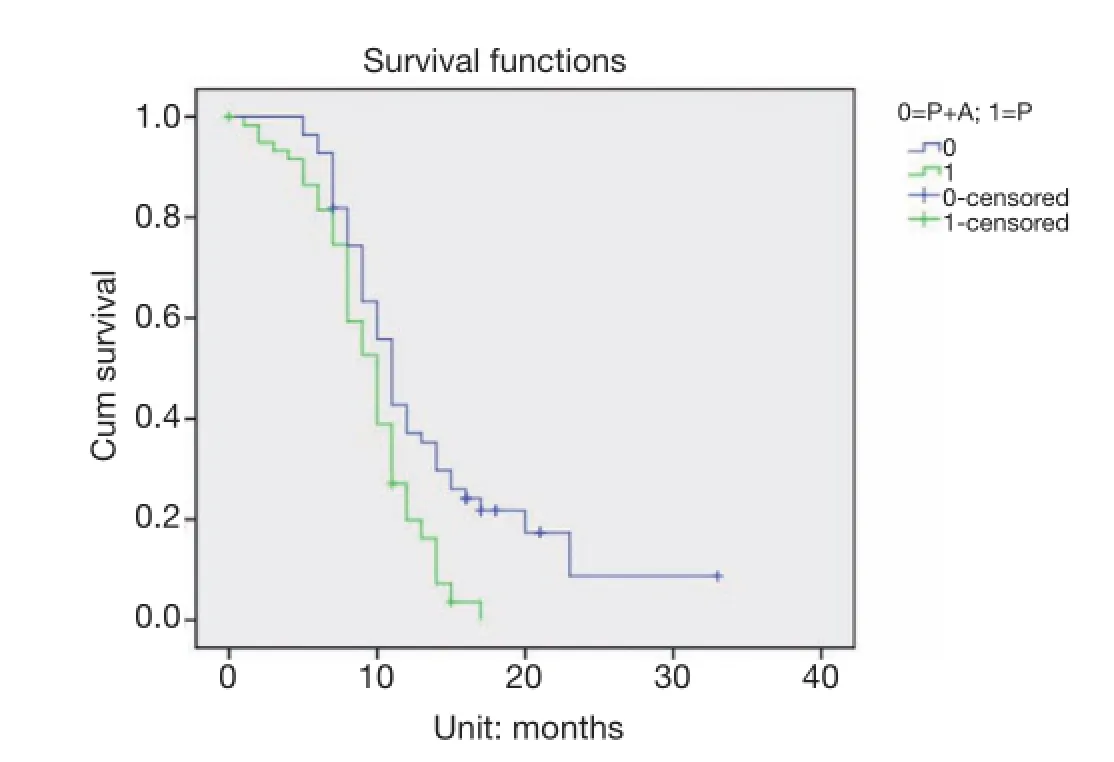
Figure 1The progression-free survival (PFS) times of patients in the third-line pemetrexed monotherapy group (P) and pemetrexed plus bevacizumab double-drug combination group (P + A) were 9.49 and 13.64 months, respectively (P=0.001).
The median PFS values of patients with third-line treatment in the pemetrexed monotherapy group and pemetrexed in combination with bevacizumab group were 9.49 and 13.64 months, respectively (P=0.001) (Figure 1). The median OS was 36.22 and 38.76 months, respectively (P=0.04) (Figure 2).
Subgroup analysis of the third-line pemetrexed plus bevacizumab dual-drug combination group in 55 cases showed that the long-term efficacy of first-line GP and second-line EGFR-TKI in 29 cases of patients (median PFS and OS were 17.00 and 42.80 months, respectively) was superior to that of first-line EGFR-TKI and secondline GP in 26 cases of patients (median PFS and OS were 9.70 and 34.46 months, respectively) (P<0.05). Additionally, subgroup analysis of the pemetrexed monotherapy group of 61 cases showed that the long-term effcacy of frst-line GP and second-line EGFR-TKI in 34 patients (median PFS and OS were 13.80 and 38.72 months, respectively) was better than that of first-line EGFR-TKI and secondline GP in 27 patients (median PFS and OS were 8.80 and 32.94 months, respectively) (P<0.05) (Table 2).
Discussion
In the current retrospective study of 116 cases of EGFR mutation-sensitive patients with the application of GP chemotherapy and EGFR-TKI in a different firstand second-line order and the third-line application of pemetrexed with or without bevacizumab, the impact of different medication orders and combinations of these lines of drugs on patient outcomes was preliminarily investigated.
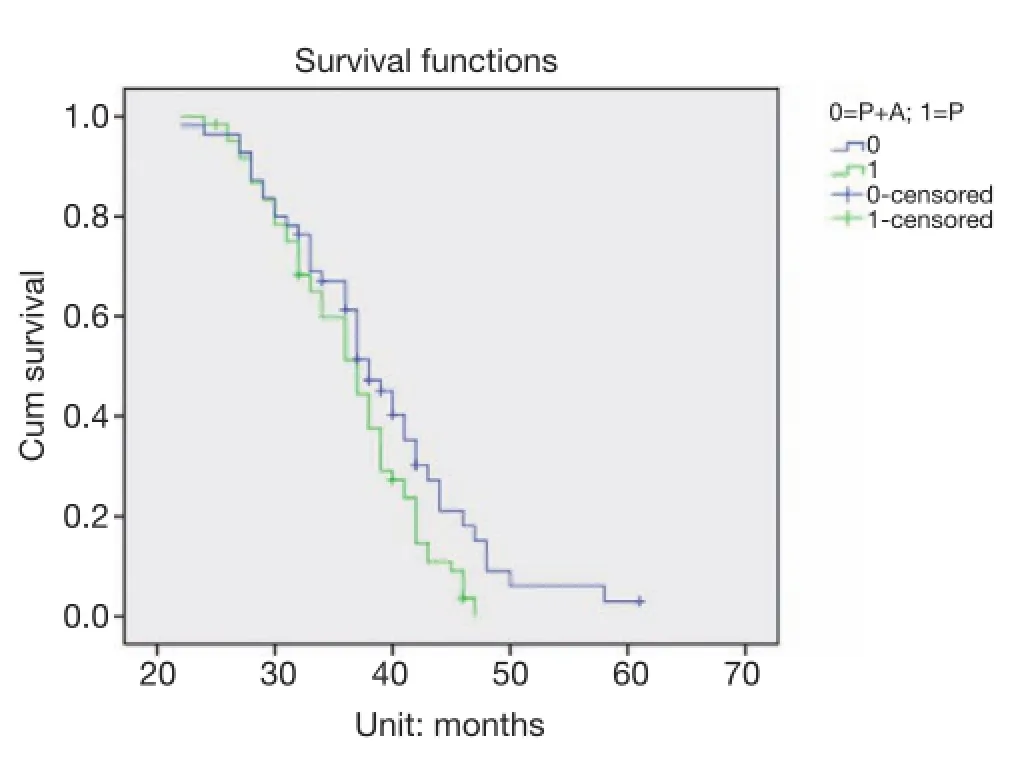
Figure 2The median overall surviva time of third-line pemetrexed monotherapy (P) in 61 cases was 36.22 months, the median survival time of third-line pemetrexed plus bevacizumab (P + A) in 55 cases was 38.76 months (P=0.04).
For patients with advanced EGFR mutation-sensitive lung cancer, EGFR-TKI treatment must be considered. In 2004, it was reported that very promising results were achieved after the administration of gefitinib in a patient with ineffective multi-line chemotherapy; this case study set the prelude to targeted therapy use in lung adenocarcinoma (12). Subsequent studies including IPASS, NEJ002, WTJOG3405, EURTAC and OPTIMAL (3-5,13,14), all found in subgroup analysis that for EGFR mutation-sensitive patients, regardless of frst-line EGFRTKI or first-line chemotherapy, the survival time was the longer in patients who had received both treatments. However, how to choose third-line medication after second-line treatment, or whether the order of the former two-line therapy would affect the third-line therapy, has not yet been reported.
Pemetrexed was originally used for the treatment of pleural mesothelioma (15) and has achieved remarkable results. Subsequent studies have also confrmed its position in the treatment of lung adenocarcinoma (16-18). Bevacizumab, a recombinant vascular endothelial growth factor (VEGF) monoclonal antibody, is the world’s first VEGF inhibitor approved for marketing and may be combined with all VEGF isoforms (19). In terms of the range optimization of treatment, for patients with advanced EGFR mutation-sensitive lung adenocarcinoma, after frstor second-line use of three generations of cisplatin dual-drug and EGFR-TKI, third-line bevacizumab may be a choice for the treatment plan. Studies on bevacizumab and pemetrexed have indicated that both drugs may have advantages in lung adenocarcinoma and are safe with low toxicity (16,20). The previous studies indicated that the application of the pemetrexed and bevacizumab combination regimen maybe a better choice as a thirdline treatment for patients with a poor performance status (PS) score and advanced EGFR mutation-sensitive lung adenocarcinoma (20).
In the current study, we found that regardless of the order of first- and second-line chemotherapy and TKI therapy, as long as bevacizumab was added to the third-line therapy, the pemetrexed in combination with bevacizumab regimen was superior to pemetrexed monotherapy. At present, or even in the future, it may be difficult to carry out prospective studies on multi-line treatment for patients with advanced EGFR mutation-sensitive lung cancer in terms of full-range optimized therapy. However, we may be inspired from the existing frst- or second-line so-called alternate treatment patterns. Similar results were obtained from previous studies (21,22) as well as this multi-line retrospective study. The reason can be explained from a biological evolution perspective. When the patients are continuously treated with the same class of drugs, due to the evolution of tumor cells, which have adapted to this environment, the tumor cells are more resistant to the same type of treatment; however, when an entirely different treatment approach is applied, due to the tumor cells failing to adapt to the new environment, the possibility of resistance may be lower. Over time, however, when the tumor cells have adapted to the new environment and become resistant and a treatment approach similar to the prior environment is re-applied, then the old treatment has to some extent become a new environment; thus, an alternative treatment strategy may be a better choice.
In conclusion, regardless of the order of the first- and second-line chemotherapy and TKI therapy, the pemetrexed plus bevacizumab regimen was superior to the pemetrexed monotherapy as the third-line therapy in patients with advanced EGFR mutation-positive lung adenocarcinoma. However, this strategy is worth further investigation in prospective studies.
Acknowledgements
Funding: This study was funded by National Key Scientifc & Technology Support Program: Collaborative innovation of Clinical Research for chronic obstructive pulmonary disease and lung cancer (NO. 2013BAI09B09).
Disclosure: The authors declare no confict of interest.
1. Cooper WA, Lam DC, O'Toole SA, et al. Molecular biology of lung cancer. J Thorac Dis 2013;5 Suppl 5:S479-90.
2. Bos M, Gardizi M, Schildhaus HU, et al. Activated RET and ROS: two new driver mutations in lung adenocarcinoma. Transl Lung Cancer Res 2013;2:112-21.
3. Mok TS, Wu YL, Thongprasert S, et al. Geftinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
4. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as frst-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42.
5. Mitsudomi T, Morita S, Yatabe Y, et al. Geftinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8.
6. Savas P, Hughes B, Solomon B. Targeted therapy in lung cancer: IPASS and beyond, keeping abreast of the explosion of targeted therapies for lung cancer. J Thorac Dis 2013;5 Suppl 5:S579-92.
7. de Lima Araujo LH. A new brainwave in non-small cell lung cancer: driving targeted therapy to central nervous system metastases. Transl Cancer Res 2013;2:51-3.
8. Obara P, Pu Y. Prognostic value of metabolic tumor burden in lung cancer. Chin J Cancer Res 2013;25:615-22.
9. Shimizu K, Okita R, Nakata M. Clinical signifcance of the tumor microenvironment in non-small cell lung cancer. Ann Transl Med 2013;1:20.
10. Bar J, Urban D, Borshtein R, et al. EGFR mutation in lung cancer: tumor heterogeneity and the impact of chemotherapy. Chin Clin Oncol 2013;2:2.
11. Aggarwal C. Targeted therapy for lung cancer: present and future. Ann Palliat Med 2014;3:229-35.
12. Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to geftinib. N Engl J Med 2004;350:2129-39.
13. Inoue A, Kobayashi K, Maemondo M, et al. Updatedoverall survival results from a randomized phase III trial comparing geftinib with carboplatin-paclitaxel for chemonaïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9.
14. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as frst-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
15. Nowak AK. Chemotherapy for malignant pleural mesothelioma: a review of current management and a look to the future. Ann Cardiothorac Surg 2012;1:508-15.
16. Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97.
17. Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51.
18. Zukin M, Barrios CH, Pereira JR, et al. Randomized phase III trial of single-agent pemetrexed versus carboplatin and pemetrexed in patients with advanced non-small-cell lung cancer and Eastern Cooperative Oncology Group performance status of 2. J Clin Oncol 2013;31:2849-53.
19. Strickler JH. Bevacizumab with frst-line chemotherapy for Medicare patients with metastatic colorectal cancer: do the risks outweigh the benefts? Transl Gastrointest Cancer 2013;2:39-43.
20. Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after frst-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous nonsmall-cell lung cancer: AVAPERL (MO22089). J Clin Oncol 2013;31:3004-11.
21. Wu YL, Lee JS, Thongprasert S, et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol 2013;14:777-86.
22. Yoshimura N, Okishio K, Mitsuoka S, et al. Prospective assessment of continuation of erlotinib or geftinib in patients with acquired resistance to erlotinib or geftinib followed by the addition of pemetrexed. J Thorac Oncol 2013;8:96-101.
Cite this article as:Zhou CZ, Qin YY, Xie ZH, Zhang JX, Ou-Yang M, Li SY, Chen RC. Effcacy of third-line pemetrexed monotherapy versus pemetrexed combination with bevacizumab in patients with advanced EGFR mutation-positive lung adenocarcinoma. Chin J Cancer Res 2014;26(6):705-710. doi: 10.3978/j.issn.1000-9604.2014.12.19
10.3978/j.issn.1000-9604.2014.12.19
Submitted Oct 12, 2014. Accepted for publication Dec 11, 2014.
 Chinese Journal of Cancer Research2014年6期
Chinese Journal of Cancer Research2014年6期
- Chinese Journal of Cancer Research的其它文章
- Comparison of three mathematical prediction models in patients with a solitary pulmonary nodule
- Application of CUSA Excel ultrasonic aspiration system in resection of skull base meningiomas
- Knockdown of HMGB1 improves apoptosis and suppresses proliferation and invasion of glioma cells
- Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance
- Impact of jejunostomy during esophagectomy for cancer on health related quality of life
- Factors contributing to lymph node occult metastasis in supraglottic laryngeal carcinoma cT2-T4 N0M0 and metastasis predictive equation
