A preliminary report on adjuvant analgesic efficacy of HANS in opioid tolerant patients with cancer pain
Xiaomei Li, Jianhua Zhu, Pingping Li, Guangqing Zhu, Xiaoming Wu, Huoming Chen, Huixia Zhao, Wei Wang, Ying Zhang, Wenhua Xiao, Duanqi Liu
1Department of Medical Oncology, the First Affliated Hospital of Chinese PLA General Hospital, Beijing 100048, China;2Department of No.3 Cadre Ward; Expert Group, Beijing Army General Hospital, Beijing 100700, China;3Key Laboratory of Carcinogenesis and Traditional Research (Ministry of Education), Department of Integrated Traditional Chinese and Western Medicine, Peking University Cancer Hospital & Institute, Beijing 100142, China;4Department of Medical Oncology, the General Hospital of the Air Force of the Chinese PLA, Beijing 100142, China;5Integrated Department, Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CAMS), Beijing 100021, China;6Department of Medical Oncology, the Second Artillery General Hospital, Beijing 100088, China
Correspondence to: Wenhua Xiao. Department of Medical Oncology, the First Affliated Hospital of Chinese PLA General Hospital, Beijing 100048, China. Email: w_hxiao@hotmail.com.
A preliminary report on adjuvant analgesic efficacy of HANS in opioid tolerant patients with cancer pain
Xiaomei Li1,2, Jianhua Zhu1, Pingping Li3, Guangqing Zhu4, Xiaoming Wu5, Huoming Chen6, Huixia Zhao1, Wei Wang3, Ying Zhang3, Wenhua Xiao1, Duanqi Liu2
1Department of Medical Oncology, the First Affliated Hospital of Chinese PLA General Hospital, Beijing 100048, China;2Department of No.3 Cadre Ward; Expert Group, Beijing Army General Hospital, Beijing 100700, China;3Key Laboratory of Carcinogenesis and Traditional Research (Ministry of Education), Department of Integrated Traditional Chinese and Western Medicine, Peking University Cancer Hospital & Institute, Beijing 100142, China;4Department of Medical Oncology, the General Hospital of the Air Force of the Chinese PLA, Beijing 100142, China;5Integrated Department, Cancer Institute and Hospital, Chinese Academy of Medical Sciences (CAMS), Beijing 100021, China;6Department of Medical Oncology, the Second Artillery General Hospital, Beijing 100088, China
Correspondence to: Wenhua Xiao. Department of Medical Oncology, the First Affliated Hospital of Chinese PLA General Hospital, Beijing 100048, China. Email: w_hxiao@hotmail.com.
Objective:To observe the adjuvant analgesic efficacy of Han's Acupoint Nerve Stimulator (HANS) in opioid tolerant patients with cancer pain.
Methods:A prospective non-controlled study was conducted. Opioid tolerant patients with cancer pain were enrolled and treated with both routinely analgesics and adjuvant HANS (2/100 Hz for 30 min/d, 5 d on and 2 d off for two weeks). Cancer pain, quality of life (QOL), anxiety and depression were assessed before enrollment and on d 8 and d 15 with the BPI-C, EORTC QLQ-C30, and self-rating anxiety scale (SAS)/ self-rating depression scale (SDS), respectively; the therapeutic frequency of breakthrough pain (BP) and daily opioid dose were also recorded.
Results:Totally 47 patients meeting the inclusion criteria participated in this study; 43 patients completed the two-week treatment and assessment. The mean scores of patient's "worst" and "least" pain intensity assessed with BPI-C decreased significantly on d 8 and d 15; the therapeutic frequency of BP also significantly decreased; but the average daily dose of opioids did not change significantly. For the nine symptoms in EORTC QLQ-C30 assessment, the mean scores of pain, fatigue, constipation and insomnia were signifcantly lower on d 8 and d 15 compared with baseline; the mean scores of the overall health status, nausea/vomiting and the incidence rates of both anxiety and depression also decreased signifcantly on d 15.
Conclusions:To opioid tolerant patients with cancer pain, adjuvant treatment with HANS could improve pain release and patients' QOL by decreasing the severity of pain, fatigue, constipation, insomnia and other concomitant symptoms; it could also decrease the incidence rates of anxiety and depression.
Cancer pain; opioid tolerance; Han's Acupoint Nerve Stimulator (HANS)
View this article at:http://www.thecjcr.org/article/view/3695/4572
Introduction
Cancer pain is one of the most common symptoms in cancer patients and is also an important factor affecting the quality of life (QOL) (1). Although 80-90% of cancer pain can be relieved with analgesics theoretically, inadequate treatment of cancer pain is still a widespread worldwide phenomenon and approximately 50% cancer pain was not well controlled (2,3).
Opioid tolerance (4) and persistent constipation (5) are the two important factors hindering effective analgesic treatment when patients receive long-term opioid therapy. Thus, delaying opioid tolerance and relieving constipationare important to improve analgesic efficacy. Currently, the commonly adopted strategies are to reduce the opioid increments by adding adjuvant analgesics and relieve constipation by alternating various kinds of laxatives, but both methods increased the patient's medication burden without ensuring the analgesic efficacy. Searching for effective complementary and alternative methods is a feasible approach.
In the feld of complementary and alternative medicine (CAM), transcutaneous electrical nerve stimulation (TENS) is generally acknowledged as an effective adjuvant analgesic technique (6). The effcacy of TENS is induced mainly by the release of endogenous opioid peptides (7). In the 1990s, Professor Han and his team reported that low-frequency (2 Hz) electro-acupuncture (EA) stimulation caused the brain to release endorphins and the spinal cord to release enkephalins while high-frequency (100 Hz) stimulation caused the spinal cord to release dynorphins (8,9); they also found that 2/100 Hz (dense-and-disperse, DD) mode of stimulation was able to induce simultaneous release of the above three opioid peptides to produce synergistic analgesic effects (10-11). On basis of the above study results, Han's Acupoint Nerve Stimulator (HANS) was successfully developed. The advantage of HANS resides in its ability to further improve the analgesic efficacy of TENS through non-invasive acupoint electrical stimulation.
Both TENS and HANS have achieved satisfactory adjuvant analgesic efficacy for the treatment of noncancer pain (12,13), and some studies confirmed that the acupuncture-like TENS (AL-TENS) resulted in better analgesic efficacy (14); but very few reports on the application of these two methods in the treatment of cancer pain are available. Therefore, large-sample, randomized controlled trials (RCTs) are urgently needed for a comprehensive assessment of the analgesic efficacy of HANS. Before launching any RCT of HANS-based adjuvant treatment, we first carried out an uncontrolled prospective study to observe the efficacy of HANS more comprehensively and to provide the basis for optimizing the design of subsequent studies.
Patients and methods
Inclusion and exclusion criteria
Inclusion criteria: (I) pathologically confrmed malignancies (clinical diagnosis was adequate for hepatocellular carcinoma and pancreatic cancer); (II) 18-80 years of age; (III) Karnofsky performance status (KPS) score ≥40; (IV) the expected survival time ≥1 month; (V) persistent cancer pain≥1 month; (VI) ongoing opioid therapy and resulted opioid tolerance; (VII) conscious, and able to read, write and complete the survey questionnaires; and (VIII) the patients themselves agreed to participate in this study.
Exclusion criteria: (I) pregnant or lactating women; (II) hyperpyrexia; (III) cognitive impairment or communication disorders; (IV) severe functional abnormalities of the heart, liver and kidneys; (V) radiotherapy of the pain sites, systemic chemotherapy or other anticancer therapies one week before enrollment and during the study; (VI) placement of pacemakers; (VII) local infective infammation and ulcers or dysesthesia/paresthesia at the electrode attachment skin sites; and (VIII) poor patient's compliance.
This study was approved by the Ethics Committee of the First Affliated Hospital of the General Hospital of Chinese PLA. All patients involved in the study have signed the written informed consent forms.
Analgesic regimen
Routine analgesic therapy
All patients were routinely receiving opioids therapy and were opioid tolerant before enrollment. After the enrollment, they were asked to continue their past analgesic regimes and their physicians decided the dose adjustment of analgesics according to the patient's pain intensity change during the study period.
Adjuvant analgesic therapy
HANS100A analgesic apparatus (Beijing Huayun Ante technology Co., Ltd., China) was applied for adjuvant analgesic therapy using the selected acupoints as follows: one pair of electrodes was placed on Hegu (LI-4) and Laogong (PC-8) while another pair of electrodes was placed on Zusanli (ST-36) and Sanyinjiao (SP-6); 2/100 Hz DD mode of stimulation with escalating intensity (0-30 mA) to gradually adjust to the patient's maximum tolerated value. Each treatment lasted for 30 min, once daily, for two successive weeks during which a 2-day interval was designed for every five successive days of treatment (totally ten times).
Baseline data
The investigators recorded each patient's socio-demographic,clinical characteristics, and the data of analgesics they were taking. The converted daily oral morphine equivalent (OME) was calculated accordingly (15).
Survey measures
The analgesic efficacy, QOL and anxiety/depression were assessed 1 d before treatment and on d 8 and d 15 after adjuvant HANS treatment.
Cancer pain
The Chinese version of brief pain inventory (BPI-C) was used for cancer pain assessment. This scale was developed to assess the pain intensity and function interfered by pain. BPI-C, validated by Wang et al. (16), demonstrated excellent reliability and validity. The coeffcient of internal consistency (coefficient alpha) of the two dimensions, namely the pain intensity and daily life interfered by pain, was 0.89 and 0.92, respectively.
To comprehensively assess pain status and the efficacy of HANS, the observation of breakthrough pain (BP) was added on basis of BPI-C. Because no generally acknowledged scale for the assessment of breakthrough cancer pain was currently available (17) and the poor overall performance status of these patients made it difficult to complete multiple scales, we only recorded the frequency of analgesic therapies for BP 3 d before treatment and daily after treatment.
QOL
European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) (18) is a widely used scale for cancer patients; the Chinese version of EORTC QLQ-C30 localized by Zhao et al. (19) was first validated in patients with gynecological cancers. Subsequently, Wan et al. (20) verifed the Chinese version of EORTC QLQ-C30 in patients with five common types of cancers. Therefore, EORTC QLQ-C30 was used for QOL assessment in our study.
EORTC QLQ-C30 included 3 sections and totally 30 items (or questions). The actual score for each item was called the raw score (RS) and combined within the dimension in calculation if appropriate. In order to make the scores of all sections comparable, the linear transformation method was employed to convert the RS into 0-100 standard scores (SS). For Section 1 (functional items), SS=[1-(RS-1)/R]X100 (R is the full range of scores for each item); the same transformation was used for Sections 2 and 3 (symptoms and the overall health state), SS=[(RS-1)/ R]X100. Higher SS in Sections 2 and 3 indicated more severe impact on the functions and symptoms; higher SS in Section 3 indicated better health condition and QOL.
Anxiety and depression
Self-rating anxiety scale (SAS) and self-rating depression scale (SDS) were employed to assess the anxiety and depression, respectively. Each of these two scales consisted of 20 items; each item was rated 1-4 scores; the actual scores of each item were added and multiplied by 1.25 to obtain the fnal SS. The Chinese version of the two scales (21,22) has been widely used for the assessment of different populations. According to the normal criteria for the Chinese version, anxiety symptoms were present when the SAS SS >50 and depression symptoms were present when the SDS SS >53.
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was employed for statistical analysis of the study data. Quantitative data were represented as x±s; the paired t-test was used to compare the means obtained at different observation time points. Qualitative data were represented as percentage; χ2or rank sum test was used to compare the percentage changes at different observation time points. When P<0.05, the difference had statistical signifcance.
Results
Baseline data
A total of 53 cancer patients meeting the inclusion criteria were screened between December 2012 and October 2013; 47 of them agreed to participate in this study and signed the written informed consent forms; 45 patients completed 1-week treatment and observation; and 43 patients completed 2-week treatment and observation. See Figure 1 for detailed results of patient's screening, participation and withdrawal; see Table 1 for the basic information of enrolled patients in details.
Cancer pain assessment
Pain intensity

Figure 1 Patient fow. HANS, Han's Acupoint Nerve Stimulator.
The baseline data showed that the mean scores of the "worst", "average" and "present" pain intensity were 7.14±2.23, 4.57±1.32, and 4.14±1.03, respectively; only the mean score for the "least" pain intensity was <3 (2.57±1.12).
Compared with the baseline data of pain intensity, the mean scores of the "worst" and "least" pain intensity decreased signifcantly on d 8 and d 15; the reduction was more prominent with the "least" pain intensity (P<0.01); the mean score for "present" pain intensity also decreased signifcantly (P<0.01) on d 15. The "average" pain intensity was lower on d 8 and d 15, but the difference had no statistical signifcance.
Interference with daily function
In the seven items of BPI-C, pain had the greatest impact on patient's normal work with a mean score of 6.57 (SD=1.57); the mean scores of the other six items were listed in descending order as follows: walking ability, general activity, enjoy of life, sleep, mood and relations with other people.
After adjuvant treatment with HANS, the mean scores for "sleep" and "enjoy of life" improved signifcantly on d 8 and d 15; "general activities" also improved signifcantly on d 15 (P<0.05), but the improvement on d 8 was insignifcant. The remaining four items, namely the walking ability, mood, normal work and relations with other people, did not show signifcant improvement. See Table 2 for pain intensity and interference of pain with daily function before and after adjuvant treatment with HANS.
Breakthrough pain (BP)
Sixteen patients (34.04%) did not receive any extra analgesics for the treatment of BP within 3 d before enrollment; 18 patients (38.30%) were treated at least once daily; the average treatment frequency of the patients was 0.63 times/d (SD=0.71). The investigators divided the frequency of daily BP treatment into four time periods by whether the treated patients received HANS therapy on the same day for statistical analysis as follows: the first 5 d of each week (daily treatment with HANS) and the last 2 d of each week (rest days). When compared with baseline data, both the proportion of patients receiving BP treatment and the daily treatment frequency were lower in the four time periods after treatment with HNAS; all the differences had statistical significance (P<0.05) except for the mean daily treatment frequency on d 6-7 (P=0.08) (See Table 3 for the results in details).
Opioid application status
Three types of opioids were used as follows: oxycodone (sustained-release tablets), morphine (sustained-release tablets and injections) and fentanyl (transdermal patch). Oxycodone sustained-release tablets had the highest proportion of application; the proportion of application before treatment and on d 8 and d 15 after treatment was 55.32%, 57.78% and 58.14%, respectively. There was no significant difference in the mean opioid dose before andafter enrollment (P>0.05). See Table 4 for results in details.
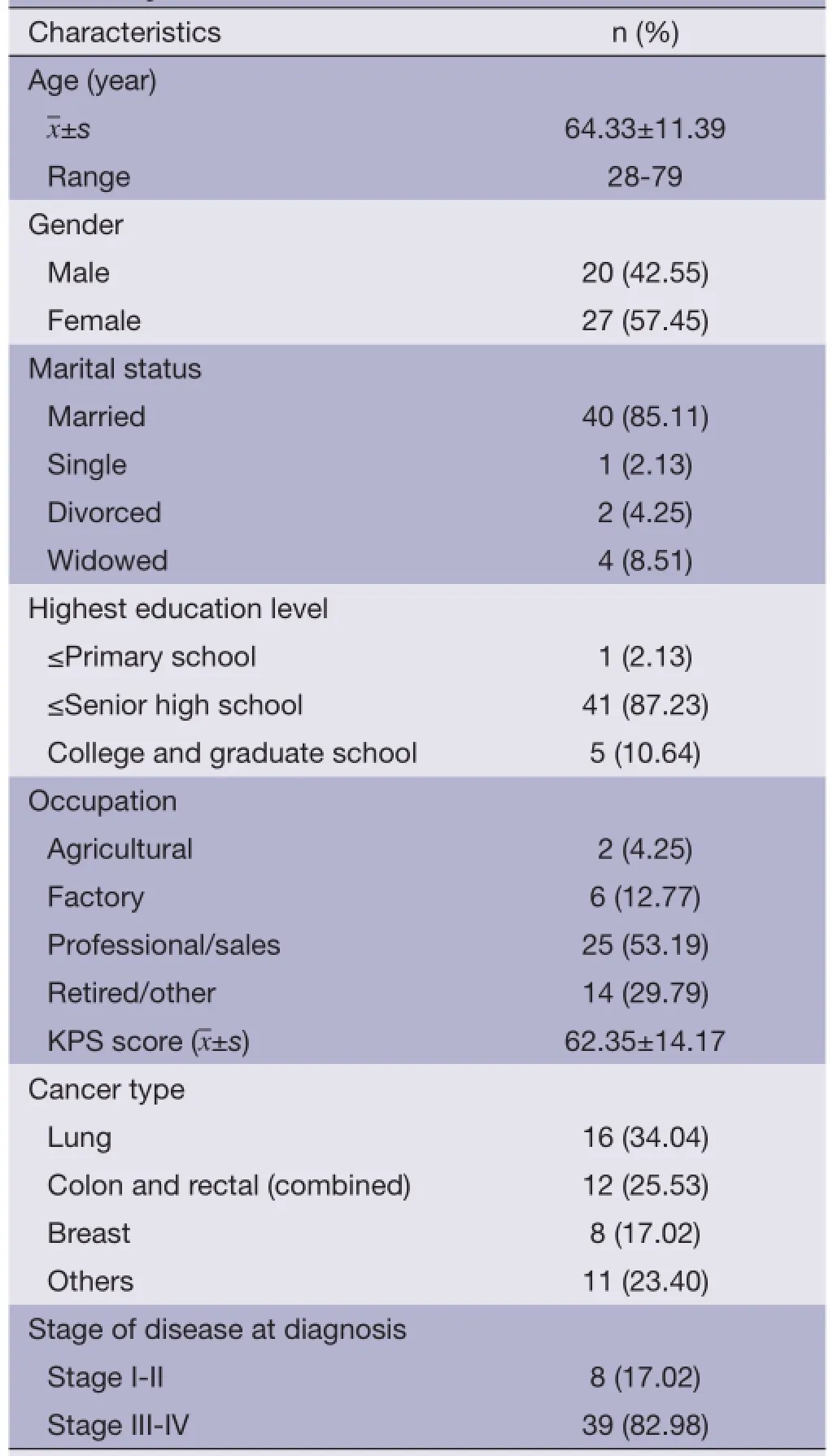
Table 1 Socio-demographic and clinical characteristics of recruited patients (N=47)
QOL assessment
EORTC QLQ-C30 assessment showed that the mean score for global health status of enrolled patients was 48.66±21.75. For the fve functional assessment dimensions, only the cognition (61.49±22.89) and mood (81.27±14.63) had mean scores >50; the mean score of physical function was the lowest, merely 37.87±19.33.
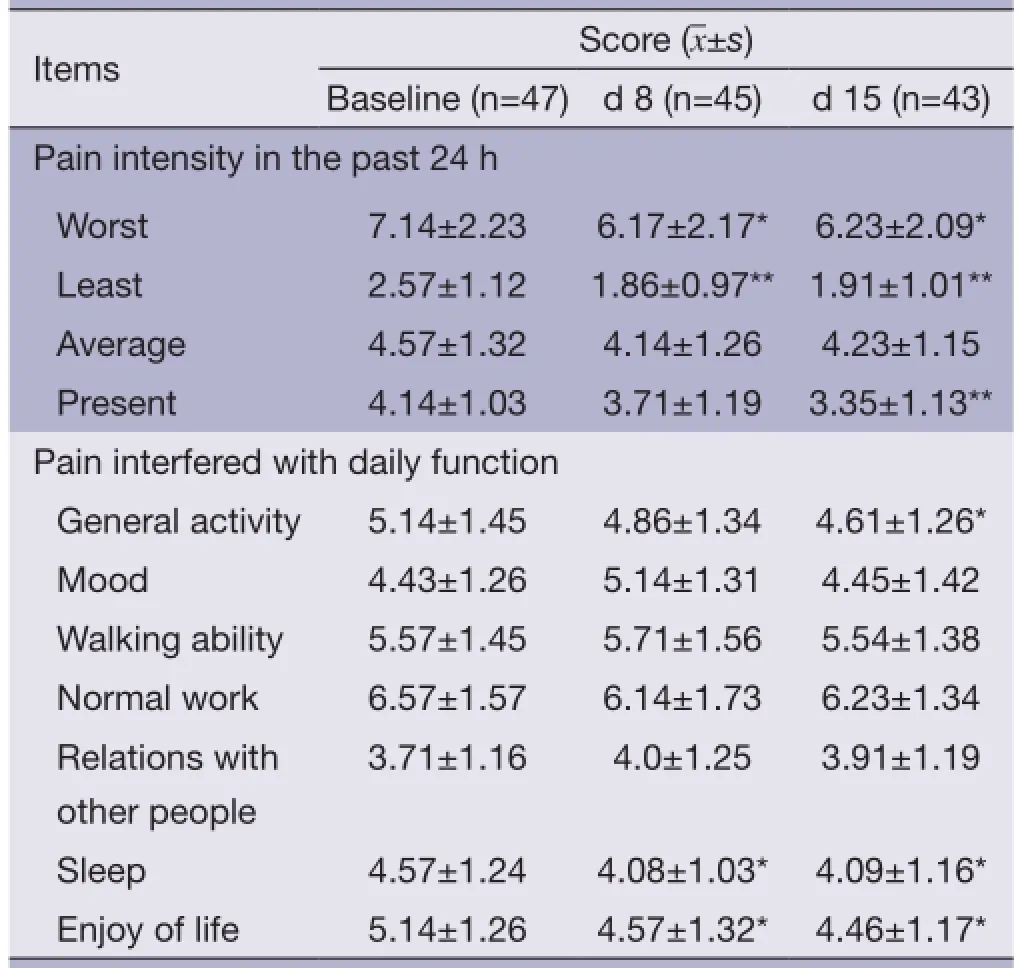
Table 2 Comparisons of pain intensity and daily function interfered on baseline, d 8, and d 15
For the nine symptom assessment dimensions, the severity of symptoms assessed by the mean scores in descending order was as follows: pain, fatigue, constipation, loss of appetite, nausea/vomiting, insomnia, dyspnea and fnancial diffculties. No patient enrolled reported diarrhea in this study.
On d 8 and d 15 of treatment with HANS, the mean scores for pain, fatigue, constipation and insomnia improved significantly when compared with the scores before treatment (P<0.05); global health status and nausea/ vomiting on d 15 also improved significantly (P<0.05); however, the mean scores for loss of appetite and dyspnea showed no signifcant changes (See Table 5 for details).
Anxiety and depression
Based on the normal criteria of SAS and SDS, 16 patients (34.04%) had anxiety symptom and 24 patients (51.06%) had depression symptoms concomitantly at the baseline evaluation. Patient's anxiety symptoms improved significantly after treatment with HANS: the mean score decreased signifcantly on d 8 and d 15; the incidence rate of anxiety (16.28%) on d 15 also decreased signifcantly; the mean score and incidence rate of depression also decreased on d 15, but the reduction on d 8 was insignificant (See Table 6 for details.).

Table 3 Comparison of frequency of BP treatment before and after HANS
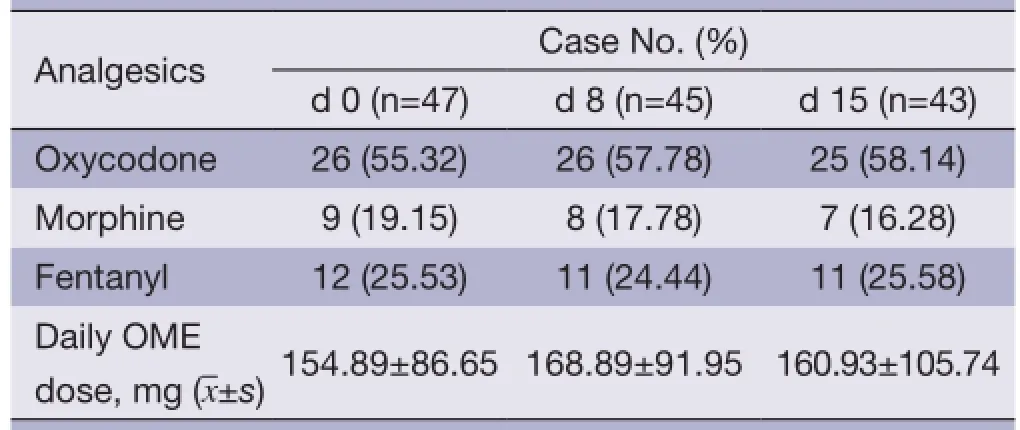
Table 4 Strong opioids prescribed of patients before and after HANS
Discussion
In this study, the baseline data indicated that 80% of the patients enrolled had advanced cancers and the overall performance status was relatively poor. In addition, the baseline BPI-C assessment showed inadequate pain release: the mean score for the "worst" pain intensity was >7; and the "average" pain intensity was >4. Pain obviously interfered with patient's daily life. Among the seven assessment items, pain had the greatest impact on normal work; except for the "relations with other people", the mean scores for the remaining 6 items were all within 4-7, which confirmed each other with the baseline QOL assessment results using EORTC QLQ-C30 and the proportion of BP treatment. In the 9-symptom items for QOL assessment, cancer pain was the most severe symptom with a mean score up to 60.82. Additionally, 38.30% of the patients were treated for BP on daily basis within 3 d before treatment; in another word, more than 1/3 of these patients experienced pain aggravation daily requiring extra opioid analgesic therapy.
The baseline assessment results of "worst" and "average" pain intensity using BPI-C in this study were very close to the data reported by scholars from Taipei, China and South Korea in 2013 (23,24), suggesting that inadequate treatment of cancer pain is still a common issue to be addressed urgently.
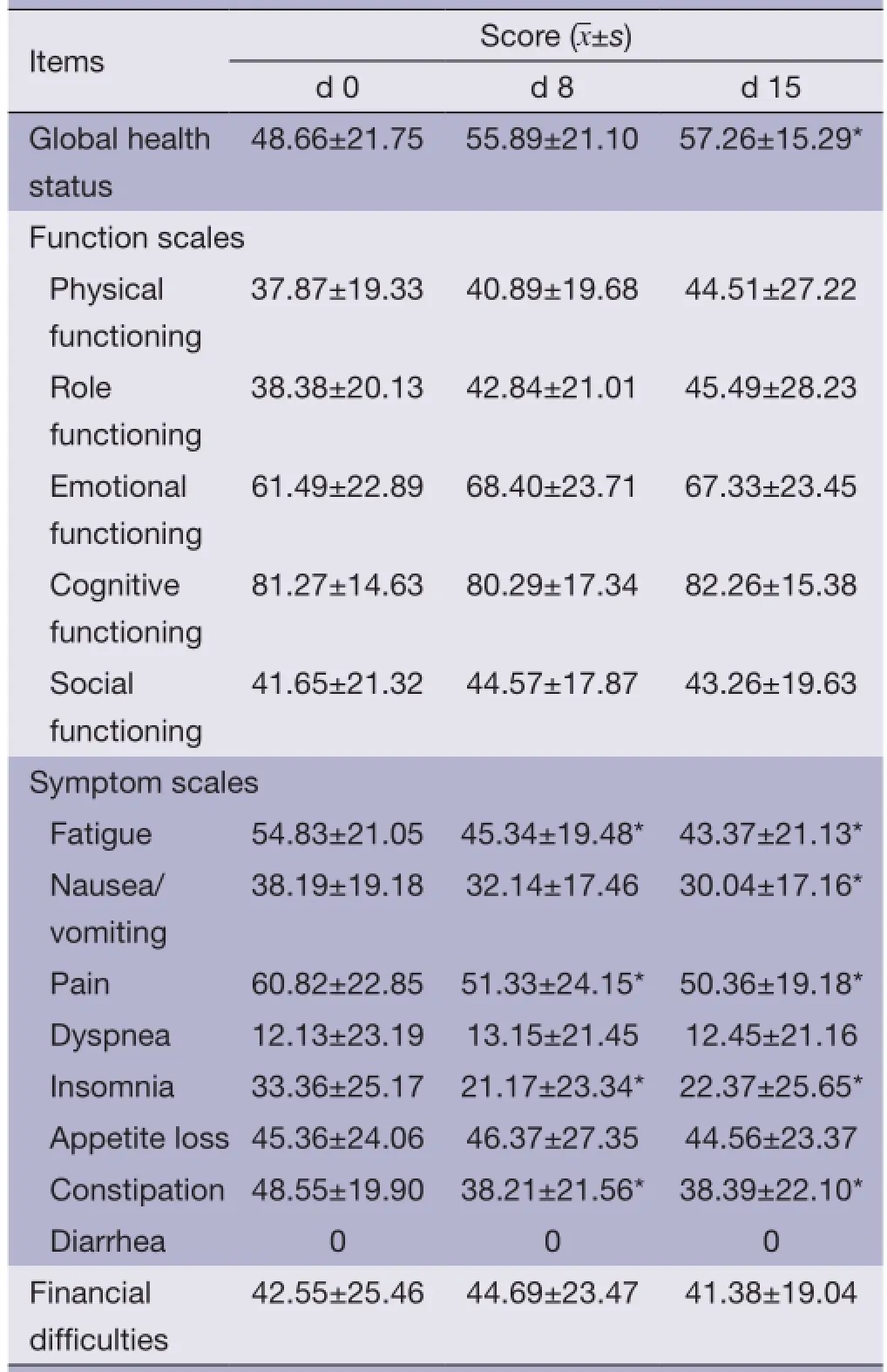
Table 5 QOL evaluation (EORTC QLQ-C30) before and after HANS
In contrast with the analgesic inadequacy is the relatively standardized analgesic therapy: more than 90% of these patients used the around the clock (ATC) dosing mode; 70% of the analgesics were taken orally; the baseline OME dose reached 155 mg/d. These results indicate that it'sfairly diffcult to carry out effective analgesic treatment in patients with advanced cancer and poor performance status by applying standard analgesic treatment alone.
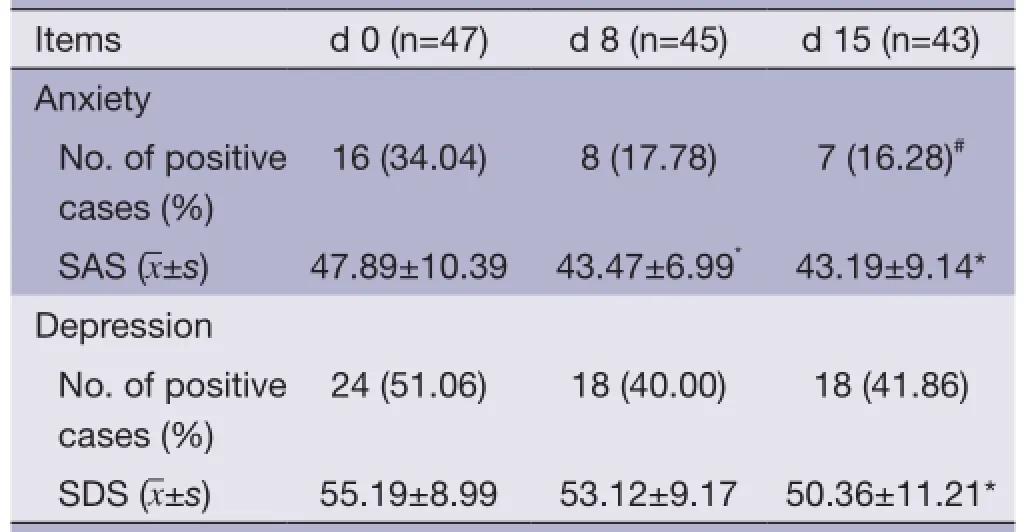
Table 6 SAS and SDS assessment before and after HANS
The efficacy assessment of adjuvant HANS therapy showed that all the three primary assessment indexes including cancer pain, QOL and anxiety/depression improved signifcantly.
Cancer pain assessment consists of three aspects, namely pain intensity, interference with daily functions and BP treatment. Three of the four pain intensity assessment items improved significantly after treatment; although the "average" pain intensity showed no significant change on d 8 and d 15, the mean scores exhibited a descending trend. Among the seven assessment items for interference of cancer pain with daily functions, three items improved signifcantly. In addition, the proportion and frequency of patients receiving treatment for BP decreased signifcantly after treatment with HANS. As a result, all the three primary assessment indexes were improved which indicated that HANS was able to relieve not only cancer pain intensity and BP but also the interference of cancer pain with daily function in these patients.
Apart from pain-related domains, this study also analyzed the impact of adjuvant HANS on patients' daily dose of opioids. The results showed no significant changes in the mean daily dose, which was inconsistent with the previous reports (25,26). Two possible factors might contribute to this inconsistency: firstly, the baseline pain status was different; the reported study subjects were mainly patients with postoperative pain but without opioid tolerance, therefore, patient's pain tend to be alleviated along with the wound healing. Secondly, the route of analgesic administration was different; effectiveness of HANS therapy was directly manifested as declined demands for opioids by the application of patient-controlled analgesia (PCA) in these studies. In contrast, cancer pain was not well controlled in this group of patients before treatment and pain might be aggravated whenever their diseases were deteriorated; the effcacy of HANS therapy was more often reflected in the improvement of pain intensity and QOL, but not in the opioid dose change. In addition, the adjustment of ATC oral opioid doses was not as convenient as PCA and cross-tolerance may exist between analgesics and HANS (27) which might attenuate the efficacy of HANS.
Apart from the analgesic effect, Chen et al. (14) also reported that AL-TENS therapy could relieve postoperative nausea and dizziness; other studies reported that acupuncture-like electrical stimulation improved the QOL in these patients (26). Given that the patients with cancer pain often have concomitant fatigue, constipation, nausea/vomiting, anxiety/depression and other symptoms, HANS therapy would have more prominent clinical application value if it can improve the symptoms and QOL on the basis of analgesic effcacy improvement. In this study, we also assessed the patient's QOL and anxiety/depression before and after HANS treatment. When compared with the patients without cancer pain reported by Wan et al. (20), this group of patients had poorer QOL and more severe symptoms, especially pain, fatigue and constipation. In addition, the proportions of anxiety and depression not only far exceeded the investigator's estimation but also were higher than the incidence rate in similar studies reported in the literature (28,29).
In this study, the patient's overall QOL improved significantly on d 15. For the nine symptom items, pain, fatigue, constipation and sleep improved signifcantly on both d 8 and d 15, among which pain and sleep improvement were consistent with the assessment results using BPI-C. The fve functional assessment items showed no significant changes before and after treatment. Despite the possible correlation with pain and other symptoms, we believe functional items may be more importantly related to tumor staging and the irreversible decline of performance status with continuous disease progression. Therefore, these items may not show signifcant changes after the relief of pain.
In addition, the assessment of patient's anxiety/depression before and after treatment with HANS showed that anxiety improved more signifcantly after treatment as evidenced by the signifcant reduction of the mean scores and incidencerate on d 8 and d 15. In our opinions, cancer pain that is not stably controlled is a persistent ever-changing stress factor related to anxiety; therefore anxiety could be relieved soon after the improvement of cancer pain. In contrast, the pathogenesis of depression is more complex and the improvement of depression is relatively more diffcult and requires longer time.
There were certain limitations of this study. First, the BP was not assessed with scales, so the provided efficacy data were limited. Second, as this was a non-controlled study, the placebo-like effect of HANS could not be eliminated.
In summary, undertreatment of cancer pain is still present in Chinese patients. This study preliminarily confirmed that adjuvant treatment with HANS could not only effectively relieve cancer pain, but also improve QOL and decrease the incidence rates of anxiety and depression by releasing pain and other concomitant symptoms. These results need randomized and controlled studies for further confrmation.
Acknowledgements
Disclosure: The authors declare no confict of interest.
1. Swarm RA, Abernethy AP, Anghelescu DL, et al. Adult cancer pain. J Natl Compr Canc Netw 2013;11:992-1022.
2. Deandrea S, Montanari M, Moja L, et al. Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 2008;19:1985-91.
3. Fisch MJ, Lee JW, Weiss M, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol 2012;30:1980-8.
4. Collett BJ. Opioid tolerance: the clinical perspective. Br J Anaesth 1998;81:58-68.
5. Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs 2003;63:649-71.
6. Claydon LS, Chesterton LS, Barlas P, et al. Dose-specifc effects of transcutaneous electrical nerve stimulation (TENS) on experimental pain: a systematic review. Clin J Pain 2011;27:635-47.
7. Henderson JM. Peripheral nerve stimulation for chronic pain. Curr Pain Headache Rep 2008;12:28-31.
8. Chen XH, Han JS. Analgesia induced by electroacupuncture of different frequencies is mediated by different types of opioid receptors: another cross-tolerance study. Behav Brain Res 1992;47:143-9.
9. Chen XH, Han JS. All three types of opioid receptors in the spinal cord are important for 2/15 Hz electroacupuncture analgesia. Eur J Pharmacol 1992;211:203-10.
10. Huang C, Wang Y, Chang JK, et al. Endomorphin and mu-opioid receptors in mouse brain mediate the analgesic effect induced by 2 Hz but not 100 Hz electroacupuncture stimulation. Neurosci Lett 2000;294:159-62.
11. Han Z, Jiang YH, Wan Y, et al. Endomorphin-1 mediates 2 Hz but not 100 Hz electroacupuncture analgesia in the rat. Neurosci Lett 1999;274:75-8.
12. Liu YL, Jin ZG. Clinical observation of the impacts and safety of electroacupuncture at Sanyinjiao (SP 6) on labor. Zhongguo Zhen Jiu (in Chinese) 2012;32:409-12.
13. Yang QH, Ma WH, Li YH. Comparison of effects of acupuncture-assisted anesthesia with different acupoint combination in gynecologic laparoscopy operation. Zhongguo Zhen Jiu (in Chinese) 2012;32:59-64.
14. Chen L, Tang J, White PF, et al. The effect of location of transcutaneous electrical nerve stimulation on postoperative opioid analgesic requirement: acupoint versus nonacupoint stimulation. Anesth Analg 1998;87:1129-34.
15. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidencebased recommendations from the EAPC. Lancet Oncol 2012;13:e58-68.
16. Wang XS, Mendoza TR, Gao SZ, et al: Chinese version of the Brief Pain Inventory (BPI-C): its development and use in a study of cancer pain. Pain 1996;67:407-16.
17. Haugen DF, Hjermstad MJ, Hagen N, et al. Assessment and classifcation of cancer BP: a systematic literature review. Pain 2010;149:476-82.
18. Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76.
19. Zhao H, Kanda K. Translation and validation of the standard Chinese version of the EORTC QLQ-C30. Qual Life Res 2000;9:129-37.
20. Wan C, Meng Q, Yang Z, et al. Validation of the simplifed Chinese version of EORTC QLQ-C30 from the measurements of fve types of inpatients with cancer. Ann Oncol 2008;19:2053-60.
21. Liang T, Liu EW, Zhong H, et al. Reliability and validity of addiction severity index in drug users with methadone maintenance treatment in Guizhou province, China. Biomed Environ Sci 2008;21:308-13.
22. Li A. Analyses on the rate and epidemic characteristics of anxiety and depression among cancer patients in Yangpu District in Shanghai. Asian Pac J Cancer Prev 2009;10:895-8.
23. Liang SY, Chen KP, Tsay SL, et al. Relationship between belief about analgesics, analgesic adherence and pain experience in taiwanese cancer outpatients. Asian Pac J Cancer Prev 2013;14:713-6.
24. Kim HS, Shin SJ, Kim SC, et al. Randomized controlled trial of standardized education and telemonitoring for pain in outpatients with advanced solid tumors. Support Care Cancer 2013;21:1751-9.
25. Lan F, Ma YH, Xue JX, et al. Transcutaneous electrical nerve stimulation on acupoints reduces fentanyl requirement for postoperative pain relief after total hip arthroplasty in elderly patients. Minerva Anestesiol 2012;78:887-95.
26. Quispe-Cabanillas JG, Damasceno A, von Glehn F, et al. Impact of electroacupuncture on quality of life for patients with Relapsing-Remitting Multiple Sclerosis under treatment with immunomodulators: a randomized study. BMC Complement Altern Med 2012;12:209.
27. Liebano RE, Rakel B, Vance CG, et al. An investigation of the development of analgesic tolerance to TENS in humans. Pain 2011;152:335-42.
28. O'Mahony S, Goulet JL, Payne R. Psychosocial distress in patients treated for cancer pain: a prospective observational study. J Opioid Manag 2010;6:211-22.
29. Twillman RK. Mental disorders in chronic pain patients. J Pain Palliat Care Pharmacother 2007;21:13-9.
Cite this article as:Li X, Zhu J, Li P, Zhu G, Wu X, Chen H, Zhao H, Wang W, Zhang Y, Xiao W, Liu D. A preliminary report on adjuvant analgesic effcacy of HANS in opioid tolerant patients with cancer pain. Chin J Cancer Res 2014;26(2):174-182. doi: 10.3978/j.issn.1000-9604.2014.04.02
10.3978/j.issn.1000-9604.2014.04.02
Submitted Jan 06, 2014. Accepted for publication Mar 14, 2014.
 Chinese Journal of Cancer Research2014年2期
Chinese Journal of Cancer Research2014年2期
- Chinese Journal of Cancer Research的其它文章
- Th22 cells are associated with hepatocellular carcinoma development and progression
- PARP inhibitor reduces proliferation and increases apoptosis in breast cancer cells
- Retrospective analysis of results of treatment for nasopharyngeal carcinoma in Macao
- Does not hUTP14a promoter form a regulation feedback loop with P53?
- Aurora-A is a novel predictor of poor prognosis in patients with resected lung adenocarcinoma
- Prognostic role of lymph node metastasis in early gastric cancer
