Effect of External Electric Field Upon Selected Proteogenic Amino Acids
Józef Mazurkiewicz; Piotr Tomasik


Abstract
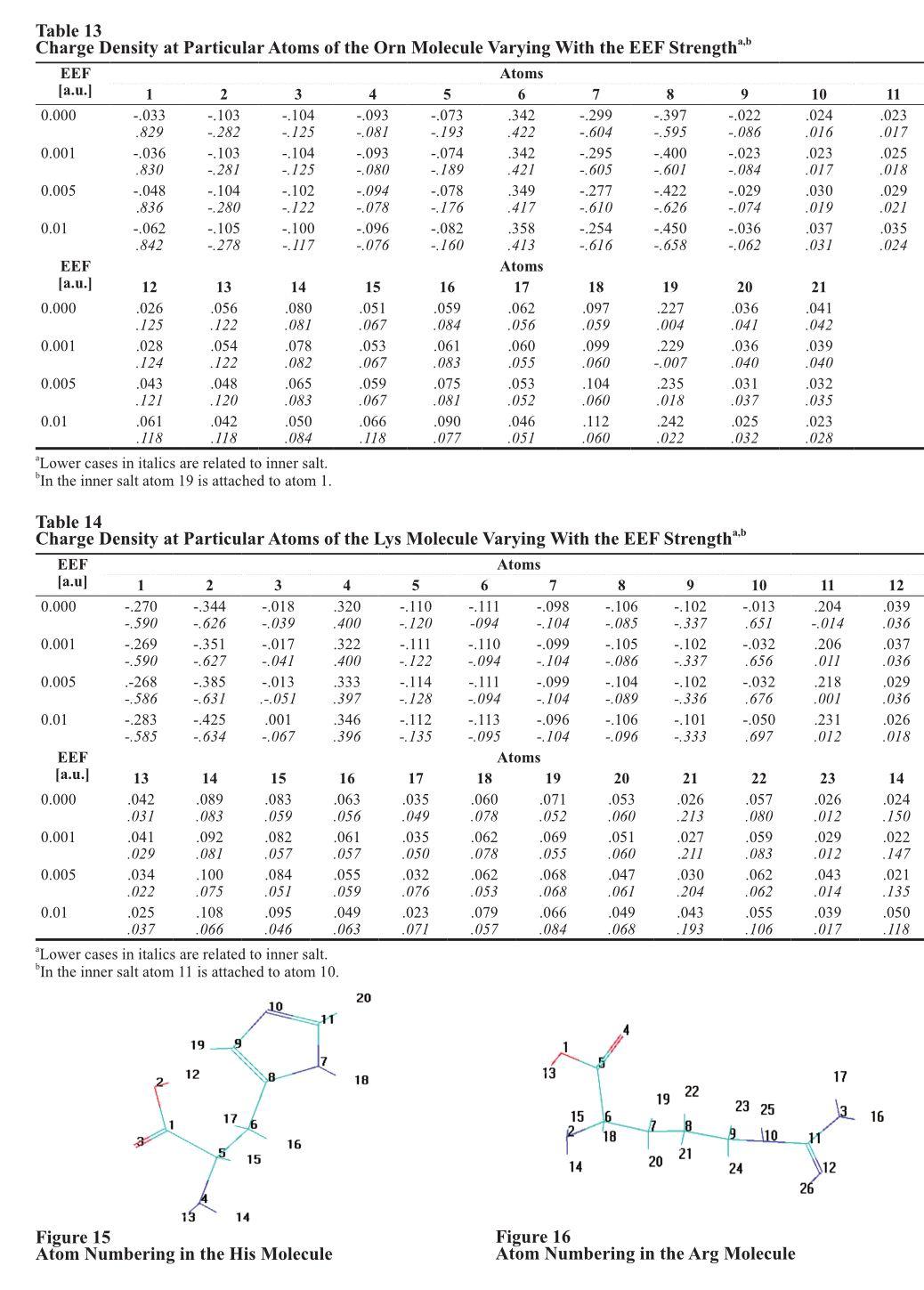
Effect of external electric field (EEF) of 0.001, 0.005 and 0.01 a.u. upon molecular energy, charge distribution and dipole moments of non-dissociated and inner salt forms of alanine (Ala), arginine (Arg), aspartic acid Asp), cysteine(Cys), glutamic acid (Gln), glycine (Gly), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), ornithine(Orn), proline (Pro), serine (Ser), threonine (Thr), tryptophan (Trp), tyrosine (Tyr), and valine (Val) were studied. For that purpose HyperChem 8.0 software was used together with the AM1 method for optimization of the conformation of the molecules in a computer vacuum. Based on the effect of EEF upon the charge density localized at the nitrogen atom of the α-amino group the acids were divided into two groups. They were Group I in which EEF increased the negative charge (Ala, Gly, Ile, Leu, Lys, Met, Phe, Pro, and Thr) and Group II in which EEF induced opposite effect (Cys, Ser, Tyr and Val). Generally, an increase in the EEF strength declined energy and increased dipole moments in non-ionized amino acids and in their inner salt forms. Energy of non-dissociated forms was more negative than these of corresponding zwitterions. Orientation of the molecules in EEF strongly depended on the EEF strength.
Key words: Alanine; Arginine; Aspartic acid; Cysteine; Glutamic acid; Glycine; Isoleucine; Leucine; Lysine; Methionine; Ornithine; Proline; Serine; Threonine; Tryptophan; Tyrosine; Valine
INTRODUCTION
Endogenous electric field controls organization of the living organisms (Pokorny, Hasek, & Jelinek, 2005). The existence of that field strongly implies an essential effect of external electric field (EEF) upon living matter. Indeed, there are numerous reports documenting such effect. Living cells absorb and convert energy of external electric field (EEF) inducing active transport of K+ and Ca2+ ions through the membranes and ATP synthesis involving ATP-synthetizes (Tsong & Astumray, 1986). That results in perturbation of active transport of ions and ATP synthesis as well as changes conformation of enzymes. Conformational changes were observed in Saccharomyces cerevisiae under the influence of pulsed EEF (Harrison, Barbosa-Canovas, & Swanson, 1997). Experiments with supersaturated aqueous glycine solutions pointed to nucleation of γ-polymorphs (Aber, Arnold, & Garetz, 2005) and such processes can take place in living cells exposed to EEF. Thus, EEF can regulate metabolism of alcohol (Crabb, Bosron, & Li, 1987; Ambroziak& Pietruszko, 1993; Berry, Grivel, & Phillips, 1993). Pulsed EEF stimulated microbial production of ethanol(Grosse, Bauer, & Berg, 1988; Nakanishi, Tokuda, Soga, Yoshinaga, & Takeda, 1988). It stimulated also synthesis of ADNP in respiration inhibited submitochondrial particles (Tiessie, Knox, Tsong, & Wehrle, 1981), citric acid production by Aspergillus niger (Fiedurek, 1999), and switching of the elevated enzymatic reactions in micelles(Harada & Kataoka, 2003). The EEF influenced also the plant growth under microgravity conditions (Nechitailo & Gordeev, 2001). Pulsed EEF was considered as the factor controlling the growth and activity of Escherichia coli and Listeria innocua in liquid food products (Dutreux et al., 2000). Papers cited above together with numerous reports on the subject describe the phenomenon and interpret it on the macromolecular level. This paper describes behavior of selected proteogenic α-amino acids placed in EEF of increasing strength. This study should be understood as introduction to understanding effect of EEF upon peptides, polypeptides and proteins as well as possibility of carrying some EEF stimulated reactions of amino acids. The applied approach was utilized in our former papers for the presentation of the effect of EEF upon simple gaseous molecules (Mazurkiewicz & Tomasik, 2010), selected monosaccharides (Mazurkiewicz & Tomasik, 2012a), lower alkanols (Mazurkiewicz & Tomasik, 2012b), porphin and metalloporphyrins (Mazurkiewicz & Tomasik, in press).
 Advances in Natural Science2013年4期
Advances in Natural Science2013年4期
- Advances in Natural Science的其它文章
- Biological Significance of Spicy Essential Oils
- Nuclear Energy From the Fission Process as an Alternative Source of Energy
- Effect of APS on Hormones Regulating Blood Glucose in Active Rats
- Spectrophotometric Determination of Methyl Paraben in Pure and Pharmaceutical Oral Solution
- Simulating Optimum Design of Handling Service Center System Based on WITNESS
- Population and Mutagenesis or About Hardy and Weinberg One Methodical Mistake
