Cloning the Dmrt1 and DmrtA2 genes of ayu (Plecoglossus altivelis) and mapping their expression in adult, larval, and embryonic stages
Jin-Hua WANG, Liang MIAO, Ming-Yun LI, Xiao-Fei GUO, Na PAN, Ying-Ying CHEN, Liang ZHAO
Ningbo University, Ningbo 315211, China
Cloning the Dmrt1 and DmrtA2 genes of ayu (Plecoglossus altivelis) and mapping their expression in adult, larval, and embryonic stages
Jin-Hua WANG, Liang MIAO, Ming-Yun LI*, Xiao-Fei GUO, Na PAN, Ying-Ying CHEN, Liang ZHAO
Ningbo University, Ningbo 315211, China
The Dmrt family of genes are involved in sex differentiation in different species of invertebrates, and some vertebrates including human. In this study, we cloned the full-length cDNA of ayu (Plecoglossus altivelis) Dmrt1 and DmrtA2. Sequence and phylogenetic tree analyses showed ayu Dmrt1 showed highest similarity to that of Oncorhynchus mykiss while ayu DmrtA2 is most similar to that of Oryzias latipes. Fluorescence-based quantitative reverse transcription PCR (qRT-PCR) revealed the Dmrt1 was predominantly expressed in the testis. At the larval stages, Dmrt1 mRNA expression level was highest during 52–64 days post hatching (dph) and at the gastrula stage during embryonic development. DmrtA2, meanwhile, was specifically expressed in the ovary and was highly expressed in the female brain tissue, but not male brain tissue. During the larval stages, DmrtA2 expression remained high before day 34, and then fluctuated while generally decreasing. During embryonic development, DmrtA2 expression increased gradually and peaked at the hatching stage. Our data suggest that ayu Dmrt1 might participate in the differentiation and maintenance of testis while DmrtA2 may play a role in ovary-differentiation and mature-ovary maintenance. DmrtA2 might also participate in brain development.
Ayu; Dmrt; Sequence analysis; Expression
The ayu (Plecoglossus altivelis; sweetfish) is a popular migratory fish and one of the most economically important freshwater fish in southeastern China and Japan due to both its perceived nutrition and taste. Belonging to the class Osteichthyes, suborder Salmonoidei, and family Plecoglossidae, the ayu is also well-known for its comparatively fast reproduction cycles. Paired with the consumer preference for the fish’s superior flavor and faint smell, the ayu has accordingly been called the “the King of Freshwater Fish” in Asia. The name may be more appropriately titles “the Queen of Freshwater Fish,” as the females reproduce more quickly and are more desirable for consumption, so much so that the sales price of female ayu is nearly double that of a male (Cao et al, 1981; Cao et al, 1982; Li et al, 1985; Wang et al, 1998).
Given the consumer preference for females and the rise in the fishes popularity in Asia, finding a way to improve the breeding production and develop selective breeding and female seeding techniques may prove worthwhile. Observational research on the wild and the cultured populations of ayu showed that the adult male and female ayu display a sexual1size dimorphism, but for this study we wanted to take a more cohesive view of the underlying dimorphism. Accordingly, in this study analyzed the gonad transcriptome to identify the members of the sex-related double-sex and Mab-3-related transcription factor (Dmrt) gene family. The Dmrt family of transcription factors, including the sexdeterminant (Dsx) of Drosophila melanogaster, and the Mab-3 of nematode, are characterized by a DNA-binding DM-domain, an unusual zinc-finger structure (Kim et al, 2003; Ren et al, 2001). Currently, at least 8 genes in the Dmrt family (Dmrt1–Dmrt8) (de Grandi et al, 2000; Guo et al, 2005; Kettlewell et al, 2000; Kondo et al, 2002; Nanda et al, 1999; Shibata et al, 2002) have
been detected across many species, including mammals (de Grandi et al, 2000), reptiles (Kettlewell et al, 2000), birds (Nanda et al, 1999), fish (Kondo et al, 2002), and amphibians (Shibata et al, 2002). A previous study indicated that the Dmrt gene family plays a major role in an organism’s sex development (Capriglione et al, 2010; de Grandi et al, 2000; Kettlewell et al, 2000; Marchand et al, 2000; Yang et al, 2013;), while another study pointed out that Dmrt participated in somite development (Seo et al, 2006).
In particular, Dmrt1, one of the prominent genes in this family, plays an important role in sex-determination and differentiation in Drosophila melanogaster, Nematodes, Oryzias latipes, and mice. To date, the regulatory mechanisms underlying sex determination and differentiation in some species has been studied. For example, knocking out Dmrt1 expression during male differentiation leads to gonad feminization in chickens (Smith et al, 2009) while in mice, the Dmrt1 gene inhibits female programming in the testis after birth (Matson et al, 2011). In some fish, Dmrt1 shows testis-specific expression (Guan et al, 2000; Liu et al, 2004; Kobayashi et al, 2004; Shin et al, 2009) while in others it shows expression in both the ovary and testis, with the expression in the testis being marginally higher than that in the ovary (Guo et al, 2004; Marchand et al, 2000). In male fish, Dmrt1 is generally expressed only in the sertoli cells, but the same is not true for females (Kobayashi et al, 2008). Meanwhile, DmrtA2 (Dmrt5), another member of the Dmrt gene family, has been identified in some fish, including the zebrafish (Guo et al, 2004), swordfish (Veith et al, 2006), and rice field eel (Zhang et al, 2006). Previous studies on DmrtA2 function mainly found relation to the brain and gonads; though in humans, the function is associated with development of the cerebral cortex (Saulnier et al, 2013) while in toads it is related to the formation of the olfactory placode nerve (Parlier et al, 2013).
Although many previous reports focused on the molecular heredity of ayu (Huang et al, 2004; Wang et al, 2011; Dong & Nobuhiko, 2003), they were restricted to the isoenzyme, biochemical heredity, and RAPD genetic diversity. To our knowledge, there are no previous studies focusing on ayu sex differentiation and its regulation. In this study, we screened the members of Dmrt gene family from the transcriptome and cloned Dmrt1 and DmrtA2. We examined their expression in different adult tissues, and during larval and embryonic developmental stages in order to provide a better understanding of the relevant mechanism at play in the sex differentiation among ayu.
MATERIALS AND METHODS
Experimental materials and reagents
Ayu fish were obtained from Qingjiang base, Zhejiang Fisheries Research Laboratory. From August-October, different tissues were sampled were frozen, immediately dissected, and then stored in liquid nitrogen at −80℃ until further analysis. The embryos of different stages were collected at 1.5 mL DNase/RNase-free centrifuge tube immediately and then stored in liquid nitrogen.
TRIzol reagent, pMD19-T vector, PrimeScript RT reagent Kit, 3′-Full RACE, and 5′-Full RACE Kit were purchased from Takara company. Other reagents were domestic analytical reagents and were purchased from Bioequip. Escherichia coli DH5α cells were stored in our lab.
cDNA cloning and amino acid sequence analysis
The total RNA of different tissues was isolated using RNAiso Plus (Takara) according to the manufacturer’s instructions. The RNA was digested by RNasefree DNase I and purified. The reverse transcription reaction was performed in a 10-μL volume using the PrimeScript RT reagent Kit (Takara). The reaction was performed at 37°C for 15 min and 85°C for 5 s, and then stored at −20°C.
Specific primers were designed for RACE and RTPCR (Table 1) based on the partial transcriptome sequence, and β-actin was used as a control. All the primers were synthesized by Sangon Biotech.

Table 1 Primers for Dmrt cloning and analysis in ayu (P. altivelis)
All procedures were performed according to users’manuals (3′-Full RACE Core Set Ver. 2.0 and 5′-Full RACE Kit, Takara). The PCR products were separated on a 2% agarose gel, cloned into the pUC-19 vector, and sequenced.
All Dmrt protein sequences from different species were aligned using the NCBI blast program (http:// blast.ncbi.nlm.nih.gov/Blast.cgi). Phylogenetic trees were constructed using MEGA5.0 (Tamura et al, 2011) using the Neighbor-joining (NJ) method, yielding an unrooted consensus tree with a 1 000 bootstrap replicates.
Dmrt expression analysis at different stages by RTPCR
To quantify Dmrt expression, we used RT-PCR according to the manufacturer’s protocols. PCR cycling conditions were adjusted to the following parameters: 95°C for 30 s, then 60°C for 30 s, and finally 72°C for 30 s for 40 cycles for Dmrt and β-actin in a 20-L volume containing SYBR Green (TaKaRa). The primers for Dmrt and β-actin are listed in Table 1. All experiments were performed in triplicate to ensure concordance and eliminate potential errors. The resulting data were analyzed via the 2-ΔΔCtmethod (Livak et al, 2001).
RESULTS
Cloning and sequence analysis of ayu Dmrt
The cloned 1684 bp Dmrt1 transcript (GenBank accession number: KC899210) consisted of an 882-bp open reading frame (ORF) that coded 293 amino acids, a 674-bp 3′-untranslated region (UTR), and a 116-bp 5′-UTR. NCBI conserved domain search (http://www. ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi ) showed that the DM-domain is represented by amino acids 27–73, and the Dmrt1 super-family conserved domain is represented by amino acids 187–246 (Figure 1).
We obtained the 2738-bp cDNA sequence of DmrtA2 (Figure 1; GenBank accession number: KF296364), The ORF (1 311 bp) coded 435 amino acids and contained the DM, DMA, and DMB conserved domains. The 3′-UTR (not including poly(A) signals) and 5′-UTR were 948 bp and 479 bp, respectively. NCBI conserved domain search (http://www.ncbi.nlm.nih. gov/Structure/ cdd/wrpsb.cgi) showed that the DM-domain included amino acids 51–96, and the DMA conserved domain included amino acids 187–246.
However, genomic analysis (data not shown) indicated that this transcript includes a 297-bp intron in its coding region, suggesting that it might represent an alternative splicing form of DmrtA2.
Similarity and phylogeny of amino acids of ayuDmrt
Alignment analysis showed that the identity between Dmrt1 of ayu and other teleostean is greater than 60%, with the most similar species being the rainbow (81.5% identity) and the least similar being medaka (62% identity). The identity between the Dmrt1 of Ayu and other vertebrates was less than 60% (Table 2). The NJ-phylogenetic tree showed that all the fish cluster into a subgroup, and the other vertebrates cluster into a separate group. In the fish subgroup, the fish from the Cypriniformes, Siluriformes, and Perciformes form a clade, and the ayu cluster with the rainbow trout (Figure 2). DmrtA2 amino acid comparison also showed a similarity greater than 70% between ayu and other teleostean, with the highest similarity being medaka (84%). The similarity with the other vertebrates was less than 60% (Table 2). The NJ-phylogenetic tree for DmrtA2 indicates that the ayu DmrtA2 cluster with the other fish and stay farther away from humans and mice (Figure 2).
Dmrt gene expression in ayu adult tissue
In different adult tissues, Dmrt1 showed the highest expression level in the testes and the lowest level in the intestine. Using the intestinal expression level as a reference, the expression levels in testis, ovary, and female spleen were 63.1, 3.2, and 1.6 times, respectively. DmrtA2 showed the highest expression in the female brain and the lowest expression in the male brain. Using the expression level from the male brain as a reference, the expression levels in the ovary, female brain, and female intestine were 1.3, 37.6, and 2.5 times of that in the male brain, respectively (Figure 3).
Dmrt expression in ayu larvae
The development of ayu larvae is a slow process (Li et al, 1985), so we accordingly sampled tissue from 10 days post hatching (dph) to 70 dph. For Dmrt1, the stages showing highest expression levels occurred between 52 dph and 64 dph. Using the expression level at 40 dph as a baseline, the expression levels at 52 dph and 64 dph were 81.5 and 88.9 times that at 40 dph, respectively. For DmrtA2, the expression levels increased from 10 to 34 dph and then decreased to a minimum,
with the exception of the expression levels at 40 dph and 52 dph. DmrtA2 expression reached its peak at 34 dph, which was 10.6 times that at 70 dph (Figure 4).

Figure 1 Sequences of ayu (P. altivelis) Dmrt1 and DmrtA2 cDNAs, codons, and amino acids
Expression of Dmrt genes in ayu embryos
In different embryonic developmental stages, ayu Dmrt1 was expressed throughout embryonic development, reaching a peak at the gastrula stage, and a low at the cleavage state. Using the cleavage state as a reference, the expression levels during the morula, blastula, gastrula, neuronal, heartbeat, incubation, and earlier alevin stages were 41.5, 65.3, 250.2, 45, 78.5, 35.1 and 9.4 times, respectively. DmrtA2 expression peaked at the earlier alevin stage, and the expression levels in the morula, blastula, gastrula, neural, heartbeat, incubation, and earlier alevin stages were 1.2, 1.4, 1.5, 7.7, 18 74.5 and 112.2 times of that in the cleavage stage, which was considered a reference (Figure 5).
DISCUSSION
Previous studies have established Dmrt1 as a sexdeterminant gene in a variety of species, and showed that its expression is sex-specific. For example, in the black porgy (Shin et al, 2009), Tilapia mossambica (Guan et al, 2000), and medaka (Kobayashi et al, 2008), Dmrt1 is expressed only in the testis. Similar to these results, the Dmrt1 expression in ayu testis was about 20 times of that in the ovary (Figure 3). However, Dmrt1 not only showsstrong expression in the testis, but also a subtle expression in the ovary of zebrafish (Guo et al, 2004), rainbow trout (Marchand et al, 2000), and Takifugu rubripes (Shen et al, 2007). Ohmuro-Matsuyama et al (2003) found that Dmrt1 is expressed in many different tissues of Medaka.In our study, we also detected low Dmrt1 expression in ayu intestine and kidney, leading us to infer that Dmrt1 not only plays an important role in testis maintenance but also may play some other yet unknown roles.
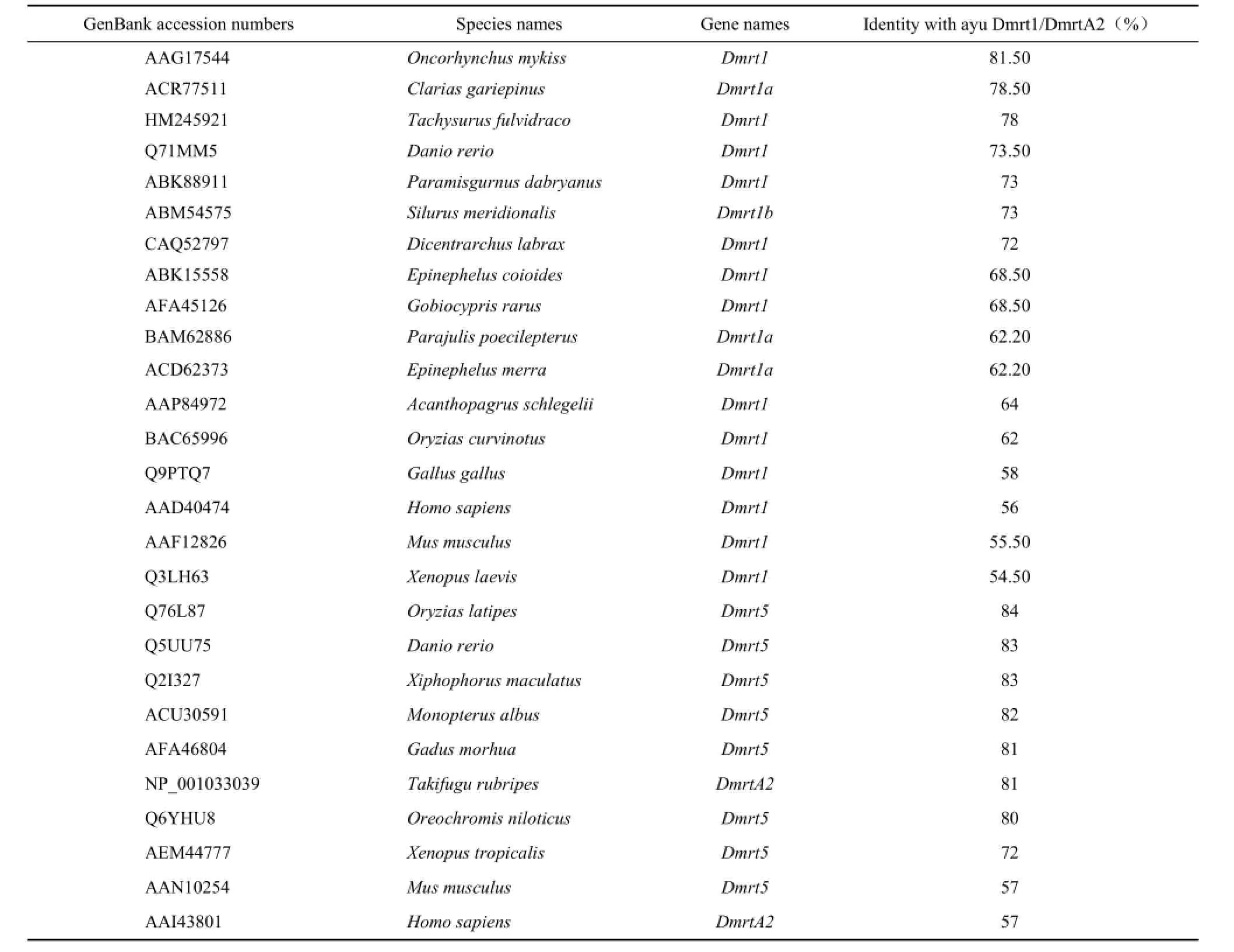
Table 2 Comparison of amino acid sequence identities of ayu Dmrt1 and DmrtA2
Compared to the adult tissues, Dmrt1 expression occurred during the entire embryonic developmental stages in both Pelteobagrus fulvidraco (Li et al, 2012) and Cynoglossus semilaevis (Sun et al, 2008). Intriguingly, the expression levels different between the two fish in that the peak expression levels occurred at a different time. During this present study, we found similar results, with the expression peak at the gastrula stage. The previous studies showed that during larval development of Pelteobagrus fulvidraco (Li et al, 2012), Dmrt1 was expressed from 1 to 51 dph and reached a peak at 31 dph, while another study (Sun et al, 2008) found that in Cynoglossus semilaevis the expression of Dmrt1 at 22 dph was higher than during the other developmental stages. In our study, Dmrt1 was expressed from 10 to 70 dph, reaching a peak at 64 dph. Moreover, examination of a paraffin section of ayu gonad revealed that the period from 40–60 dph was the key time for testis formation. During this time, Dmrt1 expression was easily detectable, implying that Dmrt1 plays an important role in the process of testis-differentiation.
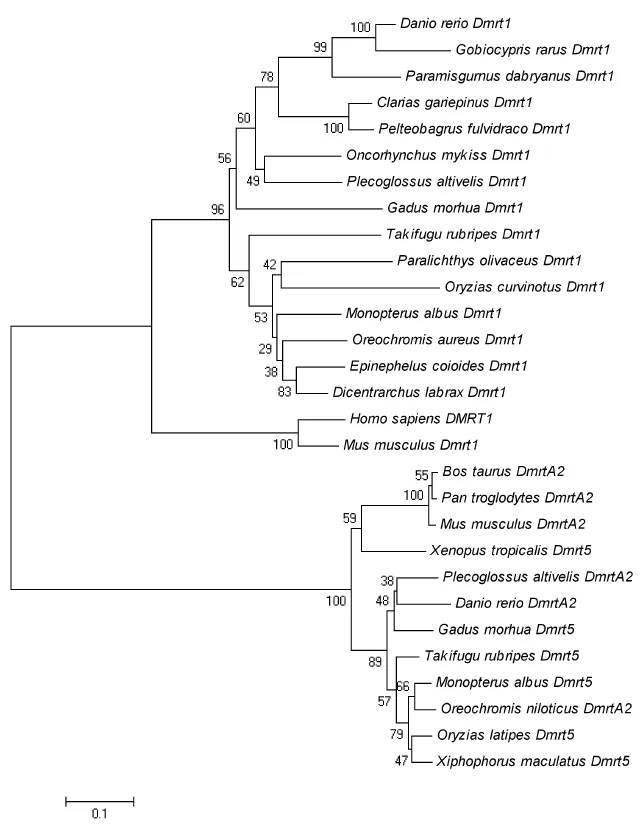
Figure 2 NJ-Phylogenetic tree based on Dmrt1 and DmrtA2 amino acid sequences Numbers at the nodes denote the bootstrap values for 1000 replicates.

Figure 3 RT-PCR analysis of Dmrt1 and DmrtA2 transcripts in different ayu (P. altivelis) adult tissues

Figure 4 RT-PCR analysis of Dmrt1 and DmrtA2 transcripts of ayu (P. altivelis) at different post-hatching developmental stages

Figure 5 RT-PCR analysis of Dmrt1 and DmrtA2 transcripts of ayu (P. altivelis) at different embryonic stages
DmrtA2 is mainly involved in the development of the brain and gonad. In mice embryos, for example, DmrtA2 is expressed primarily in both the brain and the gonads. Moreover, expression in the female ovary is higher than that in the male testis, and very lowly expressed in other tissues (Kim et al, 2003). In the puffer fish (Yamaguchi et al, 2006), DmrtA2 was expressed in the brain and eye, with no expression in gonads, while in the swordfish (Veith et al, 2006) it was expressed in the olfactory placode and midbrain and in the zebrafish (Guo et al, 2004) it was expressed primarily in the midbrain of the embryo stage. Recently, Gennet et al (2011) also found that DmrtA2 had an effect on the brain nerve in mice, a finding concordant with our results. It then stands to reason that DmrtA2 may play a vital role in the development of the ayu brain. Guo et al (2004) had previously cloned the DmrtA2 gene and found that it was expressed in the germ cells of zebrafish, mainly in the spermatogonia, spermatocytes, spermatoblasts, and oocytes, again implicating DmrtA2 in gonad development. Zhang et al (2006) elaborated on this and found that DmrtA2 was expressed in the gonad tissues of three types of rice field eels and that its expression in testis was higher than that in the ovary. And again, in the Pearlescent shellfish, DmrtA2 participates in gonad development (Yu et al, 2009). In our experiment, we found that DmrtA2 was expressed only in the ovary and had detectable expression before 34 dph, which coincides with the critical time for ovary differentiation. Considering these results, we propose that DmrtA2 likely participates in the regulation of gonad development, and may do so across numerous fish species.
In addition to influencing sex determination, Dmrt genes may be involved in the regulation of tissue development. Ayu Dmrt1 and DmrtA2 exhibited different expression patterns in females and males, but both were also expressed in a variety of other tissues, suggesting that in ayu, the Dmrt genes likely play multiple roles in organ development. Further studies may help to reveal the roles of Dmrt1 in testis development and DmrtA2 in brain development.
Cao KJ, Li MY. 1981. The preliminary study of ayu artificial reproduction. Fisheries Science & Technology Information, 9(5): 13-15. Cao KJ, Li MY. 1982. Studies on the reproductive biology of the ayu in Fuxi stream, Zhejiang. Journal of Fisheries of China, 6(2): 107-117.
Capriglione T, Vaccaro MC, Morescalchi MA, Tammaro S, De Iorio S. 2010. Differential DMRT1 expression in the gonads of Podarcis sicula (Reptilia: Lacertidae). Sexual Development, 4(1-2): 104-109.
de Grandi A, Calvan V, Bertini V, Bulfone A, Peverali G, Camerino G, Borsani G, Guioli S. 2000. The expression pattern of a mouse double sex-related gene is consistent with a role in gonadal differentiation. Mechanisms of Development, 90(2): 323-326.
Dong S, Nobuhiko T. 2003. Identification of clonal Plecoglossus altivelis using microsatellite DNA marker. Journal of Fisheries of China, 27(4): 295-299.
Gennet N, Gale E, Nan XS, Farley E, Takacs K, Oberwallner B, Chambers D, Li M. 2011. Doublesex and mab-3-related transcription factor 5 promotes midbrain dopaminergic identity in pluripotent stem cells by enforcing a ventral-medial progenitor fate. Proceedings of the National Academy of Sciences of the United States of America, 108(22): 9131-9136.
Guan GJ, Kobayashi T, Nagahama Y. 2000. Sexually dimorphic expression of two types of DM (Doublesex/Mab-3)-domain genes in a teleost fish, the tilapia (Oreochromis niloticus). Biochemical and Biophysical Research Communications, 272(3): 662-666.
Guo YQ, Cheng HH, Huang X, Gao S, Yu HS, Zhou RJ. 2005. Gene structure, multiple alternative splicing, and expression in gonads of zebrafish Dmrt1. Biochemical and Biophysical Research Communications, 330(3): 950-957.
Guo YQ, Li Q, Gao S, Zhou X, He Y, Shang X, Cheng HH, Zhou RJ. 2004. Molecular cloning, characterization, and expression in brain and gonad of Dmrt5 of zebrafish. Biochemical and Biophysical Research Communications, 324(2): 569-575.
Huang FY, Li MY. 2004. Biochemical genetic analysis of isozymes in Plecoglossus altivelis population in Fuxi. Journal of Fisheries of China, 28(5): 579-584.
Kettlewell JR, Raymond CS, Zarkower D. 2000. Temperaturedependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis, 26(3): 174-178.
Kim S, Kettlewell JR, Anderson RC, Bardwell VJ, Zarkower D. 2003. Sexually dimorphic expression of multiple doublesex-related genes in the embryonic mouse gonad. Gene expression Patterns, 3(1): 77-82.
Kobayashi T, Matsudua M, Kajiura-Kobayashi H, Suzuki A, Saito N, Nakamoto M, Shibata N, Nagahama Y. 2004. Two DM domain genes, DMY and DMRT1, involved in testicular differentiation and development in the medaka, Oryzias latipes. Developmental Dynamics, 231(3): 518-526.
Kobayashi T, Kajiura-Kobayashi H, Guan GJ, Nagahama Y. 2008. Sexual dimorphic expression of DMRT1 and SOX9a during gonadal differentiation and hormone-induced sex reversal in the teleost fish Nile tilapia (Oreochromis niloticus). Developmental Dynamics, 237(1): 297-306.
Kondo M, Froschauer A, Kitano A, Nanda I, Hornung U, Volff JN, Asakawa S, Mitani H, Naruse K, Tanaka M, Schmid M, Shimizu N, Schartl M, Shima A. 2002. Molecular cloning and characterization of DMRT genes from the medaka Oryzias latipes and the platyfish Xiphophorus maculatus. Gnen, 295(2): 213-222.
Li L, Liang HW, Li Z, Luo XZ, Zhang ZW, Zhu YY, Zou GW. 2012. Cloning and expression analysis of DMRT1 gene in Pelteobagrus fulvidraco. Journal of Huazhong Agricultural University, 31(2): 220-226.
Li MY, Cao KJ, Xu SL. 1985. A study on the development of the egg, larva and young fish of ayu (Plecoglossus altivelis T.ET S.). Journal of Zhejiang College of Fisheries, 4(1): 35-44.
Liu XS, Liang B, Zhang SY. 2004. cDNA cloning, tissue distribution and mRNA transcription of DMRT1 gene in the Protandrous Black Porgy Acanthopagrus schlegeli. Zoological Research, 25(2): 158-161. Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod. Methods, 25(4): 402-408.
Matson CK, Murphy MW, Sarver AL, Griswold MD, Bardwell VJ, Zarkower D. 2011. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature, 476(7358): 101-104.
Marchand O, Gororoun M, D’Cotta H, McMeel O, Lareyre JJ, Bernot A, Laudet V, Guiguen Y. 2000. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorchynchus mykiss. Biochimica et Biophysica Acta, 1493(1-2): 180-187.
Nanda I, Shan ZH, Schartl M, Burt DW, Koehler M, Nothwang H, Grützner F, Paton IR, Windsor D, Dunn I, Engel W, Staeheli P, Mizuno S, Haaf T, Schmid M. 1999. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genetics, 21(3): 258-259.
Ohmuro-Matsuyama Y, Matsuda M, Kobayashi T, Ikeuchi T, Nagahama Y. 2003. Expression of DMY and DMRT1 in various tissues of the Medaka (Oryzias latipes). Zoological Science, 20(11): 1395-1398.
Parlier D, Moers V, Van Campenhout C, Preillon J, Leclère L, Saulnier A, Sirakov M, Busengdal H, Kricha S, Marine JC, Rentzsch F, Bellefroid EJ. 2013. The Xenopus doublesex-related gene Dmrt5 is required for olfactory placode neurogenesis. Developmental Biology, 373(1): 39-52.
Ren LL, Cheng HH, Guo YQ, Huang X, Liu L, Zhou RJ. 2001. Evolutionary conservation of DMRT gene family in amphibians, reptiles and birds. Chinese Science Bulletin, 46(23): 1992-1996.
Saulnier A, Keruzore M, Clercq SD, Bar I, Moers V, Magnani D, Walcher T, Filippis C, Kricha S, Parlier D, Viviani L, Matson CK, Nakagawa Y, Theil T, Gotz M, Mallamaci A, Marine JC, Zarkower D,
Bellefroid EJ. 2013. The doublesex homolog DMRT5 is required for the development of the caudomedial cerebral cortex in mammals. Cerebral Cortex, 23(11): 2553-2567.
Seo KW, Wang YD, Kokubo H, Kettlewell JR, Zarkower DA, Johnson RL. 2006. Targeted disruption of the DM domain containing transcription factor Dmrt2 reveals an essential role in somite patterning. Developmental Biology, 290(1): 200-210.
Shen XY, Cui JZ, Yang GP, Gong QL, Gu QQ. 2007. Expression Detection of DMRTs and Two Sox9 Genes in Takifugu rubripes (Tetraodontidae, Vertebrata). Journal of Ocean University of China, 6(2): 182-186.
Shibata K, Takase M, Nakamura M. 2002. The Dmrt1 expression in sex-reversed gonads of amphibians. General and Comparative Endocrinology, 127(3): 232-241.
Shin HS, An KW, Park MS, Jeong MH, Choi CY. 2009. Quantitative mRNA expression of sox3 and DMRT1 during sex reversal, and expression profiles after GnRHa administration in black porgy, Acanthopagrus schlegeli. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 154(1): 150-156.
Smith CA, Roeszler KN, Ohnesorg T, Cummins DM, Farlie PG, Doran TJ, Sinclair AH. 2009. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature, 461(7261): 267-271.
Sun YY, Zhang QQ, Qi J, Wang ZG, Chen YJ, Li CM, Zhong QW. 2008. Cloning and expression analysis of DMRT1 gene in Cynoglossus semilaevis. Journal of Wuhan University, 54(2): 221-226.
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: Molecular evolutionary genetic analysis using maximum likelihood, evolutionary distance, and maximum parsimory methods. Molecular Biology and Evolution, 28(10): 2731-2739.
Veith AM, Schäfer M, Klüver N, Schmidt C, Schultheis C, Schartl M, Winkler C, Volff JN. 2006. Tissue-specific expression of dmrt genes in embryos and adults of the platyfish Xiphophorus maculatus. Zebrafish, 3(3): 325-337.
Wang GY, Ding WY, Chen SB, Xie QL, Zhou ZM, Ai WM, Wu JG. 2011. RAPD analysis on genetic diversity of four populations of Plecoglossus altivelis from Zhejiang and Fujian province. Bulletin of Science and Technology, 27(6): 863-868.
Wang HX, Li J, Xiao Y. 1998. The artificial reproduction and embryo development of ayu (Plecoglossus altivelis). Fisheries Science & Technology Information, 25(3): 30-31, 33.
Yamaguchi A, Lee KH, Fujimoto H, Kadomura K, Yasumoto S, Matsuyama M. 2006. Expression of the DMRT gene and its roles in early gonadal development of the Japanese pufferfish Takifugu rubripes. Comparative Biochemistry and Physiology. Part D, Genomics & Proteomics, 1(1): 59-68.
Yang Y, Gong P, Feng YP, Li SJ, Peng XL, Ran ZP, Qian YG, Gong YZ. 2013. Temporospatial expression of Dmrt1 in chicken urogenital system (Gallus gallus) using whole mount in situ hybridization. Acta Biologica Hungarica, 64(2): 161-168.
Yu FF, Gui JF, Zhou L, Wang MF, Yu XY. 2009. Cloning and expression characterization of Dmrt5 in Pinctada Martensii. Acta Hydrobiologica Sinica, 2009, 33(5): 844-850.
Zhang L, Chen B, Cheng HH, Zhou JR. 2006. The cloning and expression analysis of Dmrt5 in rice fieldeel. Hubei Association for Science & Technique. Hubei: Genetic Association of Hubei Province, Genetic Association of Jiangxi, 169.
Received: 7 August 2013; Accepted: 16 December 2013
Foundation items: Special Preliminary Study of 973 (2008CB117015), Changjiang Scholars and Innovative Research Team Projects (IRT0734), Priority Themes of Major Science and Technology in Zhejiang Province (2009C12077), and the K. C. Wong Magna Fund of Ningbo University
*Corresponding author, E-mail: limingyun@nbu.edu.cn
- Zoological Research的其它文章
- Sequencing and phylogenetic analysis of the Pyrgilauda ruficollis (Aves, Passeridae) complete mitochondrial genome
- mRNA expression and DNA methylation in three key genes involved in caste differentiation in female honeybees (Apis mellifera)
- Microsatellite analysis of variation among wild, domesticated, and genetically improved populations of blunt snout bream (Megalobrama amblycephala)
- Effects of cold narcosis on memory acquisition, consolidation and retrieval in honeybees (Apis mellifera)
- Effects of hydroperiod duration on developmental plasticity in tiger frog (Hoplobatrachus chinensis) tadpoles
- Factors determining the average body size of geographically separated Arctodiaptomus salinus (Daday, 1885) populations

