BCNU/PLGA microspheres: a promising strategy for the treatment of gliomas in mice
Tongming Zhu,Yiwen Shen,Qisheng Tang,Luping Chen,Huasong Gao,Jianhong Zhu
Fudan University Huashan Hospital,Dept.of Neurosurgery,National Key Laboratory for Medical Neurobiology,Institutes of Brain Science,Shanghai Medical College-Fudan University,Shanghai 200040,China
Introduction
Gliomas,accounting for more than 40% of brain tumors,are the most common primary tumors of the central nervous system (CNS).Surgical resection and radiotherapy have provided mild survival benefit,however the chemotherapeutic strategies to treat malignant glioma,including glioblastoma multiforme (GBM),have long been limited.Two major obstacles,which restrict the efficacy of anti-tumor for gliomas,are their dose-limiting toxicities to systemic organs and poor delivery across the blood-brain barrier (BBB).Furthermore,recent efforts have been put on designing polymer devices that release anti-neoplastic drugs sustainedly into the surgical cavity of resection,which is due to the features of malignant gliomas local recurrence.
BCNU (also called as carmustine),an important chemotherapeutic alkylating agent,is able to partially cross the BBB due to its low molecular weight and good lipid solubility (1).Even so,when treated systemically,the short elimination half-life of drug increases the administration frequency,and poor selectivity for tumor tissue,would lead to severe adverse effects,such as myelosuppression,hepatic toxicity and pulmonary fibrosis (2,3).To a large extent,these prevent BCNU from being applied as a potential antiglioma drug.Local delivery via biodegradable polymers could enhance local effective drug concentration and reduce the adverse effects.Local concentrations at the implantation site in experimental models achieve 1,300 times higher concentrations compared to concentrations achieved by intravenous administration of BCNU (4).Approved by FDA for the treatment of glioma in 1996,BCNU-loaded polyanhydride wafer (Gliadel) is used to implant into the tumor cavity after resection (5).Although Gliadel shows a promising prospect,only three-month increase in mean survival is far from satisfaction (6).Additionally,Gliadel may cause a series of complications,such as seizures,cerebral edema,infection and abnormal wound healing (7).
Poly (D,L-lactide-co-glycolide) (PLGA),which is capable of releasing drug in a diffusion-regulated,controlled,and hydrolyzed fashion,is a preferable biologically inert polymer among all the synthetic biodegradable polymers (8,9).Antitumor agents release period from the PLGA matrix can be modulated from days to years by different degradation period of PLGA.PLGA provides the additive advantages of being fully biodegradable without the problems of permanent implants based on non-biodegradable polymers (10).Especially,the biocompatible property of PLGA had been proven in the CNS of rodents and human (11).
In the present study,we investigated the effects of BCNU/PLGA on tumor growth,apoptosis and chemotherapy resistance in a C57BL/6 mice orthotopic brain glioma model using GL261 cell line.Our results show that local administration of BCNU/PLGA wafers improves the survival quality and time,inhibits the tumor proliferation,induces more cell apoptosis,and does not increase the chemotherapy resistance.
Materials and methods
Preparation of BCNU/PLGA wafers
All chemicals were reagent grade.PLGA,having molecular weight of 8,000 g/mol (50:50 mole ratio of lactide to glycolide),was purchased from Boehringer Ingelheim(Germany).BCNU was purchased from Sigma Chemical Co.(USA) and stored at -20 ℃.BCNU-incorporated PLGA microspheres (BCNU/PLGA) were prepared by the water-in-oil-in-water (W/O/W) emulsion technique as described previously (12).Briefly,6 mL dichloromethane containing 200 mg PLGA were mixed with 50 mg of the BCNU in 1 mL of 0.4% aqueous polyvinyl alcohol [The composition ratio of PLGA to BCNU was 4:1 (w/w)].The mixture was homogenized at 8,000 rpm for 5 min,and the resulting BCNU/PLGA emulsion was then mixed with 300 mL of 0.25% aqueous polyvinyl alcohol.The resulting mixture was homogenized with at 3,000 rpm for 3 min.In order to evaporate the dichloromethane,the fi nal BCNU/PLGA/polyvinyl alcohol emulsion was stirred gently for 3 h.The BCNU/PLGA microspheres were centrifuged at 1,000 rpm for 10 min,washed three times with deionized water,and lyophilized.One milligram of BCNU/PLGA microspheres were compression molded into wafers using Carver Press (MH-50Y CAP 50 tons,Japan) at 20 kgf/cm2for 5 s at room temperature.The wafers were 1 mm (diameter)×1 mm (thickness) in size with a flat surface and stored at 0 ℃until use.BCNU/PLGA wafers were sterilized in the clean bench prior to treatment by using UV sterilization for 30 min.
Establishment of GL261 glioma model
GL261 glioma cells were purchased from Institute of Biochemistry and Cell Biology (IBCB),Shanghai Institutes for Biological Sciences,Chinese Academy of Sciences.The cells were cultured in DMEM medium with 10% FBS,penicillin (100 units/mL),and streptomycin (100 g/mL).
Male C57BL/6 mice,6-8 weeks old and weighed 20-25 g,were used in the experiments.All surgical and experiment procedures were reviewed and approved by the Animal and Ethics Review Committee.C57BL/6 mice were anesthetized by injection of diazepam (5 mg/kg) and ketamine (50 mg/kg)intraperitoneally,and then immobilized on a stereotactic head frame (Thermo Fisher Scientific,IL,USA).A midline incision was made on the scalp and a burr hole was drilled 2.5 mm lateral to the sagittal sinus at the midpoint between bregma and lambda.GL261 cells (4×105cells in 10 μL PBS) were injected to a depth of 2.5 mm from the bone window by using a microsyringe for 10 min,and then the wound was closed.
Implantation of BCNU/PLGA wafers
A total of 60 mice with tumor were equally randomized into three groups after intracranial tumor implantation.Group A served as an untreated control; Group B was treated with PLGA on postoperative day (POD) 14; Group C was treated with BCNU/PLGA wafers (1 mg) as interstitial chemotherapy on POD 14.On POD 14,all GL261 tumorbearing mice of Groups B and C were anesthetized as described above.Around the burr hole which was made previously,we performed a small cranioectomyy,and then made a cruciate incision into the dura.We aspirated the underlying brain tissue over the tumor gently,and exposed the surface of the tumor.In Groups B,we covered the surface of GL261 tumor with 1 mg PLGA.In Groups C,1 mg BCNU/PLGA wafers containing 0.2 mg BCNU were added.

Figure 1 Kaplan-Meier survival curves of mice bearing intracranial GL261 glioma tumors in different groups.
During the treatment,mice were assessed on a daily basis,including weight change,neurological functions and survival.The neurological status scale contained eye response,level of consciousness and motor response (13).
Magnetic resonance imaging (MRI) scan
During the survival study,five mice in each group were randomly selected to evaluate the tumor growth with MRI scans on POD 7,14,21 and 28.Before MRI examination,mice were anesthetized as described above.All MRI scans were performed on 7.0T Micro-MRI (Southeast University,China).T1 weighted MRI scans were performed after gadolinium administration (0.2 mmol/kg).T2 weighted MRI scans were performed to locate.
Harvesting of specimens and Immunohistochemistry
In order to evaluate survival time,ten mice in each group were treated without sacrifice.On the 28th POD,we killed the other mice,resected the brain,sectioned axially,fixed in 10% buffered formalin,and embedded with paraffin.Paraffin-embedded tissues were used to assay Hematoxylin and eosin (H&E) staining and expression of O6-methylguanine-DNA methyltransferase (MGMT),Bax,Bcl-2 (Millipore,USA).Tissue sections (5 μm thick)were mounted on silanized glass slides,dried overnight,deparaffinized in xylene,treated with a graded series of alcohol,and rehydrated in PBS (pH 7.4).The antibody of MGMT,Bax and Bcl-2 were used to stain their respective antigens.For quantification of immunostaining,the number of stained cells was counted in ten random fields at 400×magnification.
Statistical analysis
ANOVA was used for comparisons among experimental data groups; meanwhile two-sidedt-test was specialized in comparisons among two groups.Survival curve was presented by Kaplan-Meier survival and the differences among all the groups were compared using log-rank test in SPSS software (version 11).P value of or less 0.05 indicates statistical significant in differences.
Results
BCNU/PLGA wafers implantation improves survival quality and time
For evaluating the survival quality,weight change and neurological function of mice were monitored daily after implantation of GL261 glioma cells.From day 15,the weight and the neuroscore of mice in control group started to decline rapidly.There was no significant difference in weight change and in neurological function between Group A and Group B,while the conditions of mice in Group C were much better.In treated group,the neuroscore of mice remained the highest among all the groups,and there were no noticeable decline of weight,which proved that BCNU/PLGA were safe to a large extent.Survival period of GL261 glioma-bearing mice receiving different treatments are presented in a Kaplan-Meier plot inFigure 1.In Group A and B,mice were uniformly fatal in 25-34 days (median survival: 28.5 and 30.0 days),the difference between the two treatment groups was not significant.Mice treated with BCNU/PLGA (median survival: 43.5 days) had statistical difference compared to control group (P<0.05).
BCNU/PLGA Wafers implantation inhibits tumor growth
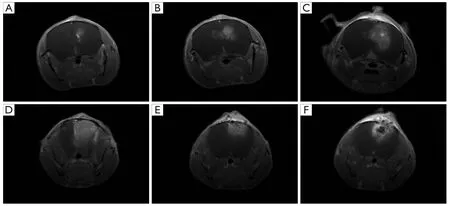
Figure 2 Magnetic resonance imaging (MRI) of GL261 glioma-bearing C57BL/6 mice,(A-D),control group; (E,F),BCNU/PLGA treated group.Images were taken on POD 7,14,21 and 28 after tumor implantation.
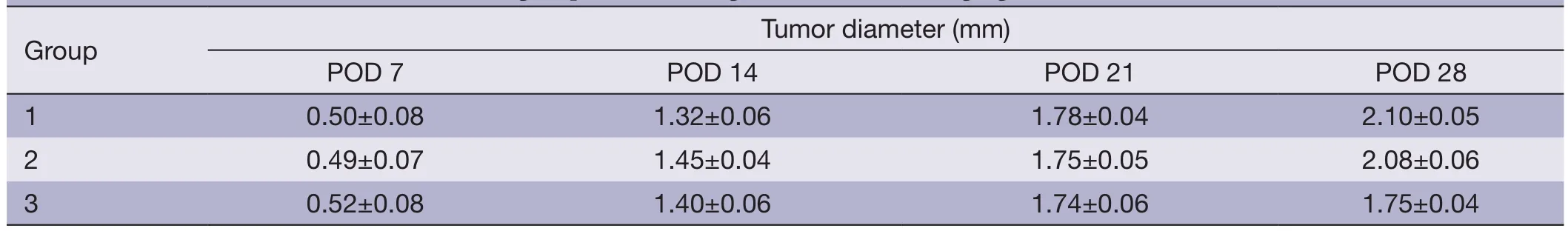
Table 1 The mean tumor diameters of each group (n=5) on Magnetic resonance imaging (MRI)
Meanwhile,fi ve mice in each group were randomly selected for observing the tumor growth using 7.0T MRI.T1 weighted MRI images enhanced by gadolinium are shown inFigure 2and the mean tumor diameters are listed inTable 1.The images of the four times scan (POD 7,14,21 and 28) showed that the size of intracranial tumor continue to increase (Figure 2A-D).With the enlargement of tumor size,necrosis and compression of cerebral ventricle could be observed in each group.On POD 28,the mean tumor diameter in control group was up to 2.10 mm,while the treatment group showed inhibitory capacity on tumor growth.The mean tumor diameter in BCNU/PLGA group remained at the size from 1.40 to 1.75 mm from POD 14 to POD 28 (Figure 2E,F),showing a statistical change comparing with control group (P<0.05).The smaller tumor diameter in the treatment group demonstrated the inhibitory capacity of BCNU/PLGA.
BCNU/PLGA wafers implantation inhibits tumor cell proliferation and induces apoptosis
The histological examination (H&E) of tumor tissues was performed on day 28 after tumor implantation (Figure 3).H&E staining showed the different proliferative levels of tumor cells in each group.The tumor revealed round nuclei with discernible chromatin staining,had uniform,dense cellularity and well vascularized with fi brillary background(Figure 3A,B).In contrast,tumors from animals treated with BCNU/PLGA showed polygonal condensed nuclei indicating apoptosis (Figure 3C).There were only a few tumor cells proliferating,which was consistent with the results of MRI,further demonstrating the best antitumor efficacy of BCNU/PLGA.
We examined the expression of Bcl-2 family-associated proteins for investigating the molecular mechanism of BCNU/PLGA-induced apoptosis of GL261 cells.Both control and treated groups showed positive expression on immunostaining.Compared to the group A and B,BCNU/PLGA decreased the expression of Bcl-2 (P<0.05),but did not elevate the expression level of Bax (P>0.05) (Figures 4,5).AS a result of immunohistochemical staining,treatment with implantation of BCNU/PLGA wafers did not alter the expression of Bax (pro-apoptotic protein) but reduced the expression of Bcl-2 (anti-apoptotic protein) in GL261 cells,thus resulted an increased ratio of Bax/Bcl-2.
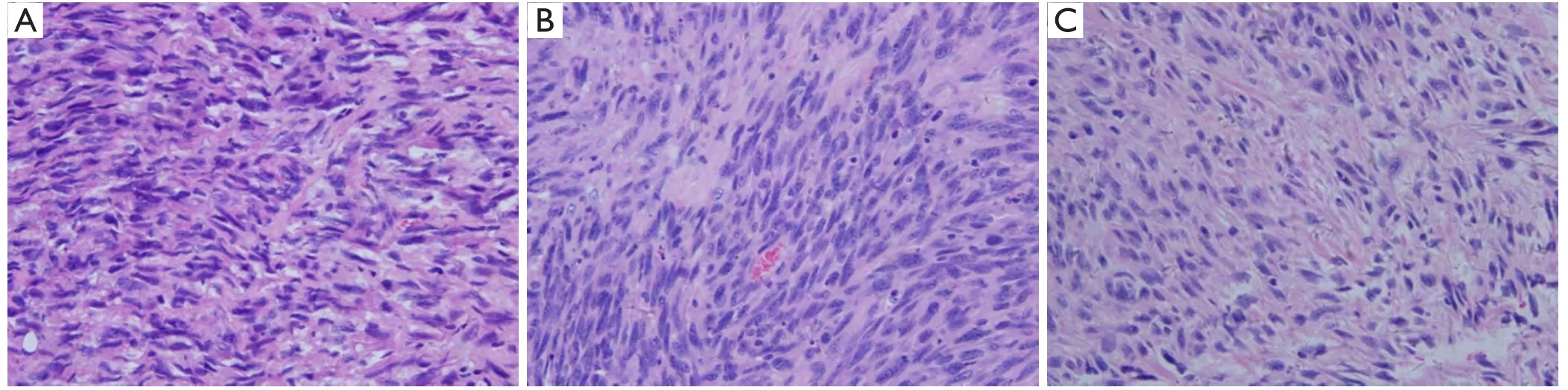
Figure 3 H&E staining of tumor tissues from GL261 glioma-bearing mice,BCNU/PLGA inhibits tumor cell proliferation (400×).
Figure 4 The immunohistochemical staining of Bcl-2 in different groups,BCNU/PLGA decreased the expression of Bcl-2 (P<0.05) (400×).
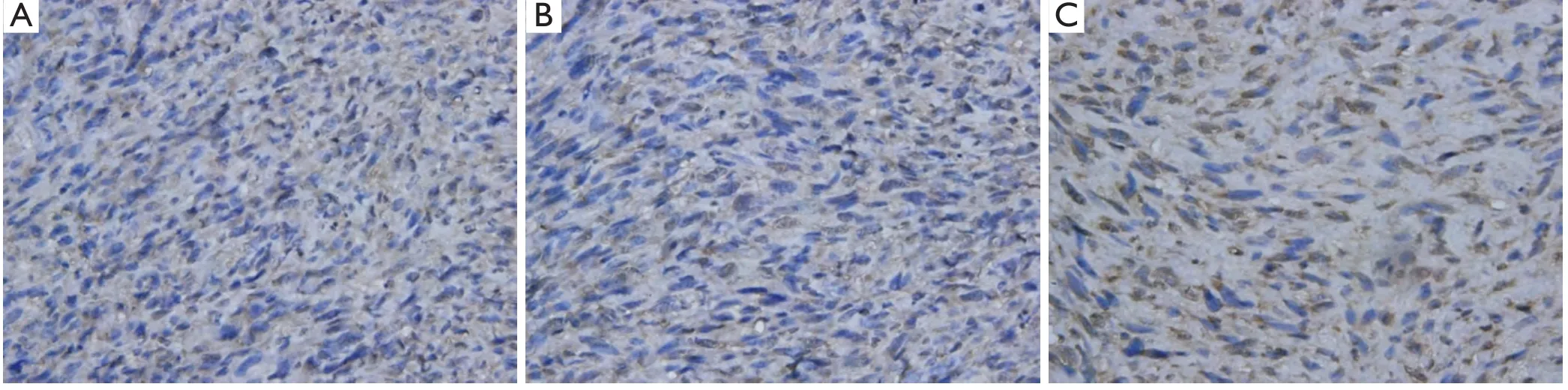
Figure 5 The immunohistochemical staining of Bax in different groups,BCNU/PLGA did not elevate the expression level of Bax (P>0.05) (400×).
BCNU/PLGA wafers implantation does not induce chemotherapy resistance
To investigate whether BCNU/PLGA regulates GL261 glioma resistance to chemotherapy,we examined the immunohistochemical expression of MGMT after Interstitial treatment of BCNU/PLGA (Figure 6).However,the immunostaining examination was non-informative.GL261 glioma cells showed a weak MGMT expression in the tumor nuclei on immunostaining of all three groups.For MGMT protein expression,no statistically signi fi cant change was found in treated group compared to control groups (P>0.05) (Figure 7).
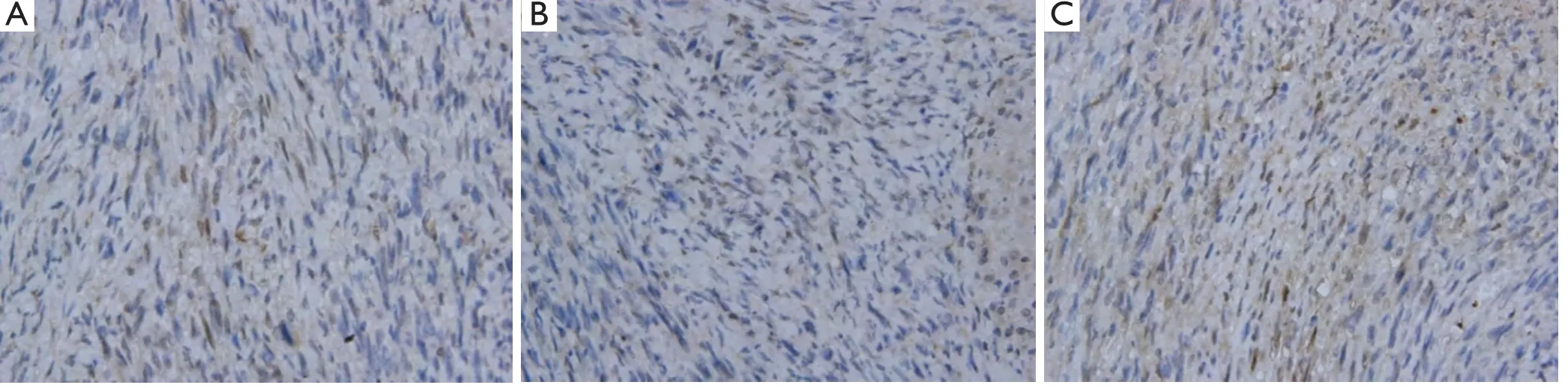
Figure 6 The immunohistochemical staining of MGMT in different groups,weak expression and BCNU/PLGA did not alter the expression level of MGMT (P>0.05) (400×).
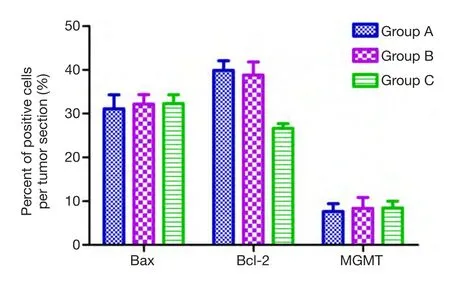
Figure 7 Percentage of positive cells of Bax,Bcl-2,MGMT in each group are shown (n=10).
Discussion
Malignant glioma,due to its infiltrative property,cannot be completely cured by surgical interference,which makes it the main cause of death from brain tumors (14,15).Due to the advantage of without passing the BBB,interstitial chemotherapy can provide high local drug concentrations,improve patient drug compliance,and minimize systemic toxicities.BCNU is an chemotherapeutic agent,which has been proved to be effective for the treatment of malignant glioma (16).PLGA has been widely used for the longterm delivery of low molecular weight drugs and several therapeutic peptides for its biodegradable and sustained release property (17).The biodegradability and biocompatibility have been demonstrated when planting the blank PLGA microspheres into brain tissue in several studies (18).We fabricated BCNU/PLGA in the present study,which can locally release antitumor drugs for a long-term period of time.No significant difference of survival time was observed between control group and blank PLGA group,the result of which suggesting that there is no inhibitory effect on GL261 cells by PLGA.The median survival time of the control,blank PLGA,and BCNU/PLGA groups in our study was 28.5,30.0,and 43.5 days,respectively.The effectiveness of interstitial chemotherapy with BCNU/PLGA has been proven in our study.The tumor volume of BCNU/PLGA treated group did not increase significantly when compared with that of control group on MRI in our study,which indicating that BCNU/PLGA could inhibit tumor proliferation.The result of survival time and histological examination is consistent with the outcome of MRI scan.
A major goal of cancer therapy is the induction of tumor cell death by apoptosis,which makes the detection of apoptosis an important diagnostic parameter in tumor tissue.The proteins in Bcl-2 family are important regulators in the mitochondrial apoptosis pathway.These oncoproteins are mainly divided into two groups: proapoptotic and anti-apoptotic (19,20).Bax is a pro-apoptotic protein,which can homodimerize or heterodimerize with other pro-apoptotic members.Bax can translate to mitochondria and insert into the outer mitochondria membrane (OMM) under an apoptotic trigger.Then the pro-apoptotic members promote the release of cytochrome C by forming mitochondrial pores,disrupting the integrity of the OMM and increasing its permeability (21,22).Bcl-2,which is one of the anti-apoptotic proteins,can protect cells by inhibiting the release of cytochrome C.These proteins interact with mitochondrial proteins including adenine nucleotide translocase (ANT) and the voltage dependent anion channel (VDAC),then prevent them from forming mitochondrial pores and keep membrane integrity (23).The ratio of Bax/Bcl-2 is therefore an important index indicating the apoptosis progression of cancer cells (24).The transcriptional and expressional Bax/Bcl-2 ratios were elevated by BCNU/PLGA interstitial chemotherapy in our present study.These findings suggest that BCNU/PLGA induces apoptosis in GL261 glioma cells by altering the expression level of mitochondrial proteins.
The major barrier to the successful treatment of malignant glioma is resistance to BCNU that is mediated by the activity of the MGMT within the tumor cells.O6position of guanine is one of the most frequent sites of DNA alkylation induced by chemotherapeutic agents.By removing these alkyl groups,MGMT,a DNA-repair enzyme,precludes the formation of crosslinks between adjacent strands of DNA (25).The expression of MGMT protects cancer cells from damages induced by chemotherapeutic alkylating agents,while protecting normal cells from carcinogens (26).Loss of MGMT expression has been reported in many tumor types,including glioma,lymphoma,prostate and breast cancer.This silencing,which preventing gene expression,is frequently related to the status of promoter methylation (25).In our study,GL261 glioma cells showed a weak MGMT expression on immunostaining of all three groups,and BCNU/PLGA treatment did not alter the expression level of MGMT protein.The result demonstrates that the interstitial chemotherapy of BCNU/PLGA does not increase the drug resistance of GL261 glioma cells.MGMT promoter methylation status is an important and vertified predictive factor of response to alkylating agents in glioma.A large number of evidence showed that glioma cells withMGMTpromoter methylation are more sensitive to BCNU(27,28).In order to figure out the mechanism of specific MGMT expression in our study,further investigations are needed to assess MGMT promoter status.
In conclusion,BCNU/PLGA improved the survival quality and time of GL261 glioma-bearing mice significantly,inhibited the tumor proliferation,induced more cell apoptosis,and did not increase the chemotherapy resistance.These results demonstrate that BCNU/PLGA local implantation could improve the efficacy of chemotherapy for malignant glioma.Our BCNU/PLGA wafer is,therefore,a promising option for the long-term interstitial chemotherapy for glioma.The results of our study provide an experimental basis for further clinical investigations of BCNU/PLGA,which could be a promising strategy for the treatment of malignant gliomas in future.
Acknowledgements
We are grateful to Dr.Hailiang Tang for paper revise,and thank Professor Wenbin Zhang of Nanjing Brain Hospital for constructive suggestions.This work was supported by grants (2010CB945500,2012CB966300,2009CB941100,81271003) from National Nature Science Foundation,Ministry of Science and Technology of China.
Disclosure:The authors declare no conflict of interest.
1.Xu X,Chen X,Lu T,et al.BCNU-loaded PEG-PLLA ultrafine fi bers and their in vitro antitumor activity against Glioma C6 cells.J Control Release 2006;114:307-16.
2.Seong H,An TK,Khang G,et al.BCNU-loaded poly(D,L-lactide-co-glycolide) wafer and antitumor activity against XF-498 human CNS tumor cells in vitro.Int J Pharm 2003;251:1-12.
3.Li Y,Ho Duc HL,Tyler B,et al.In vivo delivery of BCNU from a MEMS device to a tumor model.J Control Release 2005;106:138-45.
4.Fleming AB,Saltzman WM.Pharmacokinetics of the carmustine implant.Clin Pharmacokinet 2002;41:403-19.
5.Lee JS,An TK,Chae GS,et al.Evaluation of in vitro and in vivo antitumor activity of BCNU-loaded PLGA wafer against 9L gliosarcoma.Eur J Pharm Biopharm 2005;59:169-75.
6.Han L,Ren Y,Long L,et al.Inhibition of C6 glioma in vivo by combination chemotherapy of implantation of polymer wafer and intracarotid perfusion of transferrindecorated nanoparticles.Oncol Rep 2012;27:121-8.
7.McGovern PC,Lautenbach E,Brennan PJ,et al.Risk factors for postcraniotomy surgical site infection after 1,3-bis (2-chloroethyl)-1-nitrosourea (Gliadel) wafer placement.Clin Infect Dis 2003;36:759-65.
8.Benny O,Menon LG,Ariel G,et al.Local delivery of poly lactic-co-glycolic acid microspheres containing imatinib mesylate inhibits intracranial xenograft glioma growth.Clin Cancer Res 2009;15:1222-31.
9.Zhang YH,Zhang H,Liu JM,et al.Temozolomide/PLGA microparticles: a new protocol for treatment of glioma in rats.Med Oncol 2011;28:901-6.
10.Esther Gil-Alegre M,Gonzalez-Alvarez I,Gutierrez-Pauls L,et al.Three weeks release BCNU loaded hydrophilic-PLGA microspheres for interstitial chemotherapy:Development and activity against human glioblastoma cells.J Microencapsul 2008;25:561-8.
11.Menei P,Daniel V,Montero-Menei C,et al.Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres.Biomaterials 1993;14:470-8.
12.Ozeki T,Kaneko D,Hashizawa K,et al.Improvement of survival in C6 rat glioma model by a sustained drug release from localized PLGA microspheres in a thermoreversible hydrogel.Int J Pharm 2012;427:299-304.
13.Akbar U,Jones T,Winestone J,et al.Delivery of temozolomide to the tumor bed via biodegradable gel matrices in a novel model of intracranial glioma with resection.J Neurooncol 2009;94:203-12.
14.Ostrom QT,Gittleman H,Farah P,et al.CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006-2010.Neuro Oncol 2013;15 Suppl 2:ii1-56.
15.Zhang Y,Yao Y,Wang H,et al.Effects of salidroside on glioma formation and growth inhibition together with improvement of tumor microenvironment.Chin J Cancer Res 2013;25:520-6.
16.Paoletti P.Therapeutic strategy for central nervous system tumors: present status,criticism and potential.J Neurosurg Sci 1984;28:51-60.
17.Allison SD.Effect of structural relaxation on the preparation and drug release behavior of poly(lactic-coglycolic)acid microparticle drug delivery systems.J Pharm Sci 2008;97:2022-35.
18.Onuki Y,Bhardwaj U,Papadimitrakopoulos F,et al.A review of the biocompatibility of implantable devices:current challenges to overcome foreign body response.J Diabetes Sci Technol 2008;2:1003-15.
19.Lindsay J,Esposti MD,Gilmore AP.Bcl-2 proteins and mitochondria--specificity in membrane targeting for death.Biochim Biophys Acta 2011;1813:532-9.
20.Letai A.Pharmacological manipulation of Bcl-2 family members to control cell death.J Clin Invest 2005;115:2648-55.
21.Renault TT,Manon S.Bax: addressed to kill.Biochimie 2011;93:1379-91.
22.Degli Esposti M,Dive C.Mitochondrial membrane permeabilisation by Bax/Bak.Biochem Biophys Res Commun 2003;304:455-61.
23.D'Amelio M,Tino E,Cecconi F.The apoptosome:emerging insights and new potential targets for drug design.Pharm Res 2008;25:740-51.
24.Luo X,Budihardjo I,Zou H,et al.Bid,a Bcl2 interacting protein,mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors.Cell 1998;94:481-90.
25.Sharma S,Salehi F,Scheithauer BW,et al.Role of MGMT in tumor development,progression,diagnosis,treatment and prognosis.Anticancer Res 2009;29:3759-68.
26.Nagarajan RP,Costello JF.Epigenetic mechanisms in glioblastoma multiforme.Semin Cancer Biol 2009;19:188-97.
27.Esteller M,Toyota M,Sanchez-Cespedes M,et al.Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis.Cancer Res 2000;60:2368-71.
28.Lechapt-Zalcman E,Levallet G,Dugué AE,et al.O(6) -methylguanine-DNA methyltransferase (MGMT)promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide.Cancer 2012;118:4545-54.
 Chinese Journal of Cancer Research2014年1期
Chinese Journal of Cancer Research2014年1期
- Chinese Journal of Cancer Research的其它文章
- Prediction rule for estimating advanced colorectal neoplasm risk in average-risk populations in southern Jiangsu Province
- Long-term fatigue state in postoperative patients with breast cancer
- Diffusion-weighted images (DWI) without ADC values in assessment of small focal nodules in cirrhotic liver
- Annual report on status of cancer in China,2010
- EGFR gene copy number as a predictive biomarker for resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer treatment: a meta-analysis
- Overexpression of HOXB9 promotes metastasis and indicates poor prognosis in colon cancer
