苍耳叶挥发油化学成分及其抗肿瘤活性
刘文洁,张大帅,陈文豪,陈光英*
1海南师范大学省部共建-热带药用植物化学教育部重点实验室;2 海南师范大学化学与化工学院,海口571158
Introduction
The genus Xanthium have about 25 species widely distributed in Temperate zone,and four species and two varieties are grow abundantly throughout China including Xanthium sibiricum[1]. The whole plant of X.sibiricum,especially root and fruit,is used as medicine in China,America,and Europe.They used for their anodyne,antirheumatic,diaphoretic,diuretic,and sedative activities.According to the pharmacology research indicates that extracts of the whole plant,especially leaves,roots,fruits and seeds has antibacterial,antitumour,antitussive,antifungal,antiinflammatory,antinociceptive,hypoglycaemic,antimitotic,antioxidant,antitrypanosomal,CNS depressant activity,diuretic effects,contact dermatitis,insecticidal and herbicidal activities[2-6]. In our previous studies,its roots have the treatments of peritonitis and appendicitis,and the chloroform extracts presents the most significant effect[7].In this paper,we describe the chemical composition and antitumor activity of the essential oils from the leaves of X. sibiricum extracted by water-steam distillation to provide evidences for further development and application of the Chinese traditional herbal medicine.
Materials and Methods
Plant material
The leaves of X. sibiricum were collected from Chengmai County in Hainan Province,P.R.China in August 2012,and identified as X.sibiricum by Professor Zhong Chen from the College of Life Science in Hainan Normal University.A voucher specimen has been preserved in the Key Laboratory of Tropical Medicinal Plant Chemistry of Ministry of Education,Hainan Normal University.
Antitumor
The antitumor activity of the essential oil of leaves of X. sibiricum against BEL-7402,K562,SPCA-1,and SGC-7409 cells was evaluated by using the MTT method.
Essential oil extraction
The fresh leaves (600 g)of X. sibiricum were subjected to hydrodistillation for 6 h using using a modified Clevenger-type apparatus to produce a small amount of essential oil which was trapped in Ether. The obtained oils were dried over anhydrous sodium sulphate and stored at +4 ℃until analysed and tested.
Gas Chromatography-Mass Spectrometry (GCMS)
Analytical GC-MS system consisted of an Agilent 7890A gas chromatograph and a mass selective detector(Agilent 5975C Inert MSD). Injection was done with Agilent 7693 Series Injector (Split 20:1at 280 ℃,1.0 μL;carrier gas:helium 1. 0 mL/min;pressure:7.65psi). The MS operated in the electron impact mode with an ionization energy of 70 eV. Full scan mass spectra were acquired from 50-550 m/z.Chromatography was performed using a 30 m HP-5ms column with 0. 25 mm internal diameter and 0. 25 μm film thickness.
The detected compounds were identified by processing of the raw GC-MS data with an Agilent ChemStation data system and comparing with NIST08 mass spectral database,and from retention times and mass spectra of standard compounds. Relative amounts of detected compounds were calculated based on the peak areas of the total ion chromatograms (TIC).[8,9]
Antitumor activity assay
In the MTT assay method,the oil samples were dissolved in DMSO separately,and diluted with phosphate buffered saline (PBS)to a final concentration of 1000 μg/mL. The solutions were serially diluted with PBS containing DMSO to obtain the lower dilutions,100 μg/mL,10 μg/mL and 1 μg/mL.and stored at –70 ℃.Upon dilution in the culture medium,the final DMSO concentration was <1% DMSO (v/v).Cells were harvested at the exponential growth phase and seeded in flat bottomed 96-well microtiter plates.The cell volume in each well was 180 μL,containing 104 cells per well.The plates were incubated overnight in a 5% humidified CO2incubator at 37 ℃.The essentlal oil were then added to each well at various concentrations(100,50,10,5,1,and 0.1 μM,respectively)using a constant volume of 20 μL,in sextuplicate,while maintaining a total well volume of 200 μL.After 48-h incubation at 37 ℃in 5% CO2,50 μL of MTT (1 mg/mL of PBS)was added to each well and again incubated at 37 ℃for 4 h.After removing the medium carefully by aspiration,150 μL of DMSO was added to each well and the formazan dye crystals were dissolved by shaking gently for 15 min. The plates were then read at 570 nm wavelength in an enzyme-labeled detector(Elx800,BioTek Instruments,Inc.).IC50values of the essentlal oil in different cell lines were determined based on the dose-response curve[10].
Results and Discussion
Chemical constituents of the essential oil
The oil was analyzed by GC-MS.The identified constituents and the relative percentages were shown in Table 1 and the chromatogram were showed in Fig1.
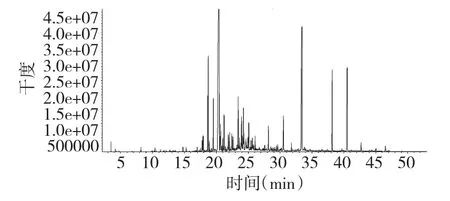
Fig.1 The total ion chromatogram of essential oil from X.sibiricum
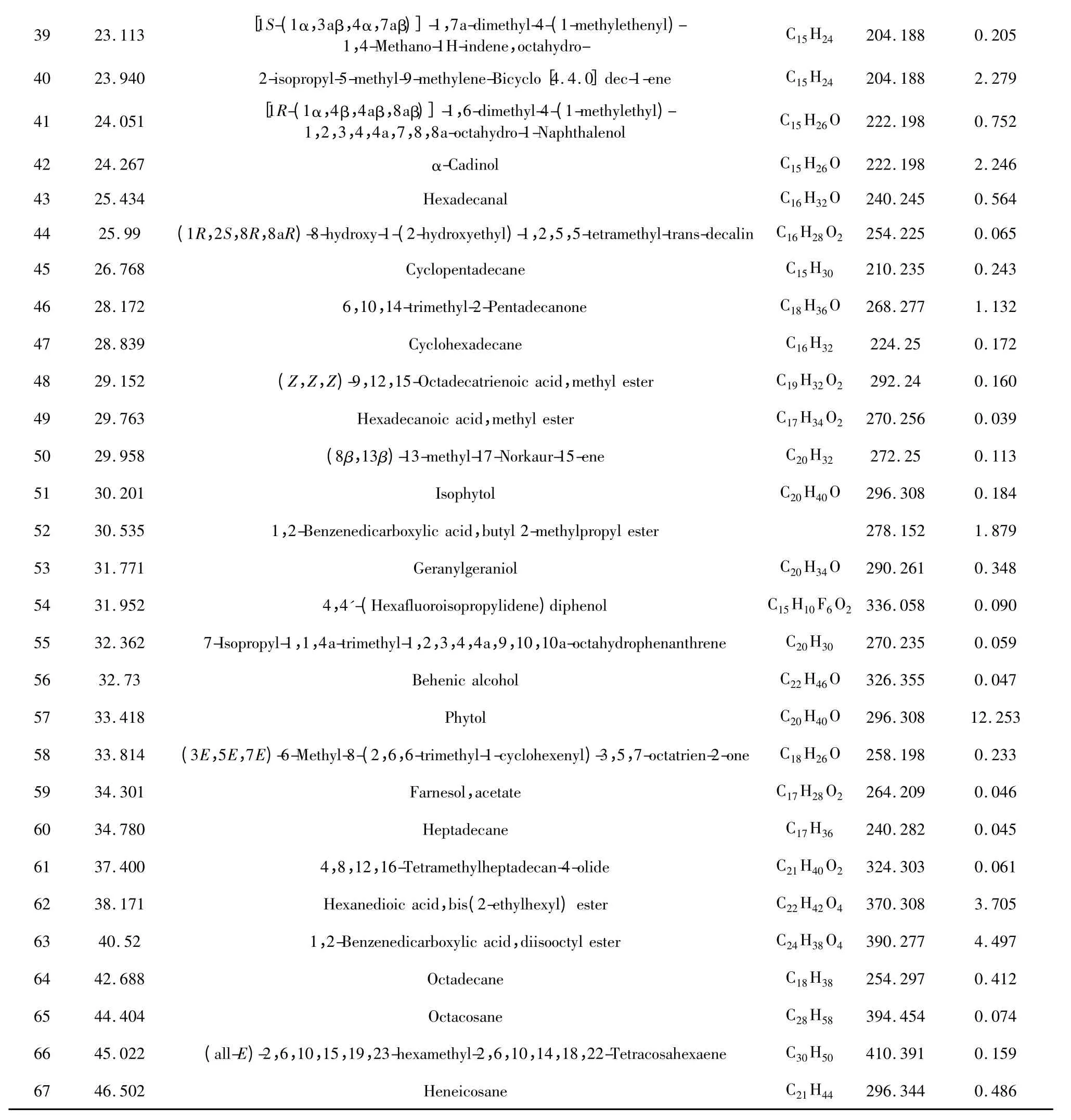
3923.113[1S-(1α,3aβ,4α,7aβ)]-1,7a-dimethyl-4-(1-methylethenyl)-1,4-Methano-1H-indene,octahydro-C15H24204.1880.205 4023.9402-isopropyl-5-methyl-9-methylene-Bicyclo[4.4.0]dec-1-eneC15H24204.1882.279 4124.051[1R-(1α,4β,4aβ,8aβ)]-1,6-dimethyl-4-(1-methylethyl)-1,2,3,4,4a,7,8,8a-octahydro-1-NaphthalenolC15H26O222.1980.752 4224.267α-CadinolC15H26O222.1982.246 4325.434HexadecanalC16H32O240.2450.564 4425.99(1R,2S,8R,8aR)-8-hydroxy-1-(2-hydroxyethyl)-1,2,5,5-tetramethyl-trans-decalin C16H28O2 254.2250.065 4526.768CyclopentadecaneC15H30210.2350.243 4628.1726,10,14-trimethyl-2-PentadecanoneC18H36O268.2771.132 4728.839CyclohexadecaneC16H32224.250.172 4829.152(Z,Z,Z)-9,12,15-Octadecatrienoic acid,methyl esterC19H32O2292.240.160 4929.763Hexadecanoic acid,methyl esterC17H34O2 270.2560.039 5029.958(8β,13β)-13-methyl-17-Norkaur-15-eneC20H32272.250.113 5130.201IsophytolC20H40O296.3080.184 5230.5351,2-Benzenedicarboxylic acid,butyl 2-methylpropyl ester278.1521.879 5331.771GeranylgeraniolC20H34O290.2610.348 5431.9524,4'-(Hexafluoroisopropylidene)diphenolC15H10F6O2 336.0580.090 5532.3627-Isopropyl-1,1,4a-trimethyl-1,2,3,4,4a,9,10,10a-octahydrophenanthreneC20H30270.2350.059 5632.73Behenic alcoholC22H46O326.3550.047 5733.418PhytolC20H40O296.30812.253 5833.814(3E,5E,7E)-6-Methyl-8-(2,6,6-trimethyl-1-cyclohexenyl)-3,5,7-octatrien-2-oneC18H26O258.1980.233 5934.301Farnesol,acetateC17H28O2 264.2090.046 6034.780HeptadecaneC17H36240.2820.045 6137.4004,8,12,16-Tetramethylheptadecan-4-olideC21H40O2 324.3030.061 6238.171Hexanedioic acid,bis(2-ethylhexyl)esterC22H42O4 370.3083.705 6340.521,2-Benzenedicarboxylic acid,diisooctyl esterC24H38O4 390.2774.497 6442.688OctadecaneC18H38254.2970.412 6544.404OctacosaneC28H58394.4540.074 6645.022(all-E)-2,6,10,15,19,23-hexamethyl-2,6,10,14,18,22-TetracosahexaeneC30H50410.3910.159 6746.502HeneicosaneC21H44 296.3440.486
The volatile oil mainly consists of alcohols,olefins,alkanes,aldehydes,esters,organic acids,The oil constituents are classified into sesquiterpene hydrocarbons (46.715%),oxygenated sesquiterpenes (4.962%)and others. Among 67 identified constituents,representing 80.370% of the oil,(1E,6E,8S)-1-methyl-5-methylene-8-(1-methylethyl)-1,6-Cyclodecadiene(28.490%),Phytol(12.253%)and Caryophyllene(5.237%)were the most abundant.The oil was characterized by the high percentage of sesquiterpene hydrocarbons.
Antitumor activity
All experiments were carried out in triplicate.The antitumor activity was assessed by evaluating the presence of IC50. Results of inhibition rate and IC50(Table 2)showed that the essential oil of X.sibiricum had different antitumor activities against the four human tumor cells tested.The anticancer test showed that the oil had inhibitory efffects on SPC-A-1 and BEL-7402.
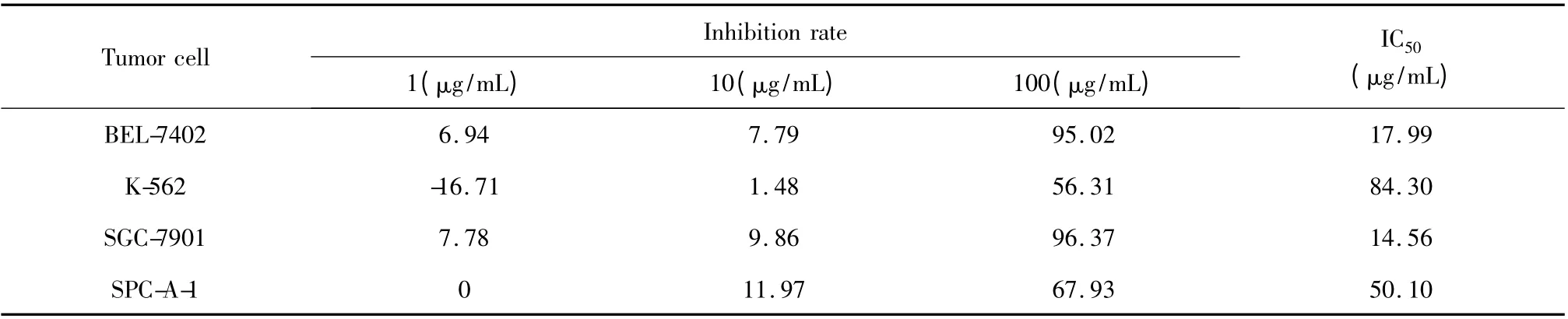
Table 2 Antitumor activity of essential oil from X.sibiricum
1 Lv YT,Hou HG,Su YH,et al. Identification of medicinal plant Xanthium L. produced in China. China J Chin Met Med.2001,26:17-20.
2 Anjoo K,Ajay KS.Phytopharmacological review of Xanthium strumarium L.(Cocklebur). Int J Gre Pharm,2010,4:129-139.
3 Kim YS,Kim JS,Park SH,et al.Two cytotoxic sesquiterpene lactones from the leaves of Xanthium strumarium and their in vitro inhibitory activity on farnesyltransferase. Planta Medica,2003,69:375-377.
4 Yadava RN,Jharbade J.Novel biologically active triterpenoid saponin from the leaves of Xanthium strumarium Linn.Asian J Chem,2007,19,1224-1230.
5 Saxena VK,Mondal,SK.A xanthanolide from Xanthium strumarium.Phytochemistry,1994,35:1080-1082.
6 Scherer RI,Duarte MCT,Catharino RR,et al.Xanthium strumarium L. antimicrobial activity and carboxyatractyloside analysis through electrospray ionization mass spectrometry.Rev Bras Pl Med,Botucatu,2009,11:159-163.
7 Kan SQ. Studies on chemical constituents from the roots of Xanthium sibiricum.Haikou:Hainan Normal University(海南师范大学),MSc.2010.
8 Li XB,Chen GY,Song XP,et al.Composition and antimicrobial activities of essential oil of leaves of Polyalthia laui Merr.Nat Prod Res Dev(天然产物研究与开发),2012,24:590-593.
9 Nasser A,Martina W,Norbert A,et al.Essential oil composition from oleogum resin of soqotraen Commiphora kua. Rec Nat Prod,2008,2(3):70-75.
10 Yang B,Chen GY,Song XP,et al.Trigoxyphins H and I:Two new daphnane diterpenoids from Trigonostemon xyphophylloides.Bioorg Med Chem Lett,2012,22:3828-3830.

