Seasonal variations in the energy budget of Elliot’s pheasant(Syrmaticus ellioti) in cage
Elliot’s pheasant Syrmaticus ellioti is a vulnerable species peculiar to China (Baillie et al, 2004), mainly distributed across the eastern hilly sub-region in central China, including Zhejiang, Anhui, Fujian, Jiangxi, Hubei,Hunan, Guangdong, and Guangxi provinces (Delacour,1977; Ding &Zhu, 1989; Li, 1996; Ding, 1998). Mainly inhabiting rugged mountains and jungle of valleys at an altitude of 200−1 500 m, more commonly in mixed conifer-broad leaved forests, Elliot’s pheasant can also be found in dense bamboo and forest understory. Elliot’s pheasant is omnivorous, mainly eating plant leaves,stems, buds, flowers, fruits, seeds and other crops, but also insects and other animals (John et al, 2000). Due to chronic deforestation, burning and vegetation reclamation, agricultural encroachment and the stress of hunting among other factors, Elliot’s pheasant is approaching of the serious loss, fragmentation and degeneration of its habitat (Ding et al, 2000).According to the survey on typical habitat of Elliot’s pheasant in Kaihua County, Zhejiang in 1984, the population density of Elliot’s pheasant was 3.5/km2in summer and 6.9/km2in winter (Li, 1985).1
Since the 1980s, domestic researchers have studied the field ecology of the form, distribution, living habits,activity regularity, breeding habits, habitat types of Elliot’s pheasant, and learned about the wild pheasant populations (Li, 1985; Long, 1985; Ding & Zhu, 1988).Since the 1990s, the “3S” technologies were used widely used for the study of the pheasant’s inhabiting features,selection mechanism as well as habitat type and feature(Ding et al, 1996, 2001; Shi & Zheng, 1997), habitat vegetation fragmentation (Ding et al, 2000) and activity area (Cai et al, 2007; Xu et al, 2007). Researchers have likewise studied more recently the genetic diversity,genetic structure and genetic flow on the basis of mitochondria DNA in Elliot’s pheasant (Jiang et al,2005a, 2005b, 2007). However, despite recent progress,the previous studies seldom deal with the energy budget and feed intake of Elliot’s pheasant (Luo et al, 2007,2011), a significant feature for studying the variation of energy ecology of Phasianidae that live in the subtropical shrub ecosystems.
The birds’ capabilities of gaining energy depend on food digestibility and energy acquisition from food in limited time, or more succinctly, only the available energy in an environment is turned into chemical energy before it can be used (Lu, 1991). Food is the basis adaptation to environmental surroundings and maintaining their lives, Elliot’s pheasant can get the energy from the everyday food, helping to keep their normal day to day activities in check and build their own bodies. Studying the energy intake of Elliot’s pheasant in cage will greatly contribute to grasping their food digestibility, and inferring the energy demand of maintaining wild Elliot’s pheasant, while also laying the basis of the population energy ecology and the environmental carrying capacity needed for successful reintroduction of Elliot’s pheasant,At the same time, this study also provides a scientific foundation for more adequate management of Elliot’s pheasant in captivity. Between March 2011 and February 2012, we researched the different seasonal energy intake of Elliot’s pheasant at different age in the Breeding Base of Endangered Pheasants, Guangxi Province - the Biological Garden of Guangxi Normal University.
MATERIALS AND METHODS
Animals
According to the mating system of the wild Elliot’s pheasant (1 male, 2 or 3 females), we selected 12 Elliot’s pheasants of different ages, that were alive and healthy,good growth and normal digestion from the Biological Garden of Guangxi Normal University in March 2011, 6 of them were 4-year old (2 males, 4 females), and once bred. The number of other group of trial pheasant were also 6 (2 males, 4 females), which be hatched in May 2010. Since Elliot’s pheasant have strong stress, in order to make the trial pheasants adapt to the environment of separate feeding, the pre-experiments had to be done before the post-experiment. Cages in which the trial pheasants lived were disinfected before every experiment preventing the specimens from being sick. There were plenty of favorite foods for Elliot’s pheasants.
Methods
The energy balance of Elliot’s pheasant was measured by the daily collection of the trial pheasants’excrement (Zhang et al, 2004). During the experiment,the birds were kept alone in a cage that consisted of an inner and outer room. The inner room (1.9 m×2.8 m) was used for feeding and rest, with one food manger hanging on the wall about 10 cm off the ground. The outer room(3.5 m×2.8 m) was used for movement, and the walls and nets of outer room were 1.25 m and 0.7 m height,respectively. The cage grounds were covered with double films. There were a variety of flowers and trees around the cage and the environment was very quiet. In order to make the trial pheasants adapt separate feeding, the preexperiment was 8 days, then, the post-experiment was 6 days. All the Elliot’s pheasants were weighed hungrily with electronic scales before and after each experiment.
The foods of Elliot’s pheasant consisted of dried corns and compound forages, which were put in the food manger, and cabbages were hung on the wall with a thin wire. The trial pheasants were fed 2 times, at 08:00 and 13:00 each day, and their excrement and urine acid were collected 3 times with the stainless steel spoons and plastic syringes, respectively, at 08:00, 13:00 and 18:00 each day, Surplus baits were collected at 18:00 each day,The food intake of every bird was weighed and calculated with the Pallet scales each day. All the collective excreta samples were mixed, signed and placed in the Petri dish, then, kept in the vacuum oven at a constant heat of 65oC for 3−4 days. The cabbages were also hung around the cage to correct the evaporation of cage’s cabbages. According to the actual situation of Guilin’s climate, the experiment times can be divided into spring (March−May), summer (June−August),autumn (September−November) and winter(December−February), and the temperatures of spring,summer, autumn and winter were 20.3, 28.0, 18.6 and 8.1oC, respectively. Energy metabolism experiments were made every month under the natural temperature.Lastly, we took the average temperature between maximum and minimum as the day temperature and the average of 6 days formal experiments as trial temperature. From January. to December in the whole year, there were 12 groups’ of temperatures were measured, which were 7.2, 5.0, 14.5, 22.0, 24.5, 26.5,28.0, 29.6, 25.2, 18.5, 12.0 and 9.0oC.
Many studies have shown there were no sex difference between food intake and excreta, and accordingly we did not take the sex difference into consideration during this experiment (Kendengh, 1970;Wang et al, 1996).
The food digestibility (%) was calculated by using the following formula: D=(Ei–Ee)/Ei, in which D is food digestibility (%), Ei is gross energy intake, Ee is excrement energy. The calorific values of corns,compound forages and Chinese cabbages were measured with GR-3500 oxygen bomb calorimeter (Changsha Instruments).
Body weight
The body weight of trial pheasants were measured with the electronic scales precise to 0.1 g. After Elliot’s pheasant nestlings were hatched by machine in May 2010 and their feather dried, their body mass were measured and regarded as 0d index; 1−30 days old nestlings body mass was recorded daily; 31± days body masses were recorded once every 2 days, 46± days old were recorded once every 3 days, 55± days old were recorded once every 5 days, 70± days old were recorded once every 10 days, until nestlings reached 100 days old.The body mass of the 4-year old adults were measured monthly, before and after the formal experiment, when the specimens were hungry.
Data analysis
Data were analyzed by using the SPSS11.5 statistical package. All results were calculated by oneway ANOVA and expressed as mean±SE. The differences of the same years' groups among different seasons were analyzed by post hoc multiple comparisons ANOVA, and the differences of the same seasons among different years’ groups were analyzed using paired t-Test,with (P<0.05) taken as statistically significant.
RESULTS
Gross energy intake, metabolic energy and food digestibility
Season gross intake, metabolic energy and food digestibility of Elliot’s pheasant were analyzed by using post hoc multiple comparisons ANOVA and paired t-Test(Table 1, Figure 1, Table 2, Figure 2), respectively. The results showed that there were significant differences among the gross energy intake of Elliot’s pheasant (4-year adults: F=9.470, P=0.000<0.05, n=72; 1-years nonages:F=9.427, P=0.000<0.05, n=72), and that the gross energy intake and metabolic energy were highest in winter and lowest in summer. In the same season, the gross energy intake of 1-year nonages was greater than for 4-year adults except spring (t=2.472, df=71, P=0.016<0.05,Table 1, Table 2). Likewise the metabolic energy of 1-years nonages was also larger than 4-year adults except for during spring (t=1.940, df=71, P=0.046<0.05, Table 1,Table 2). The average food digestibility (%) of 4-year adults and 1-years nonages were (85.44±0.54)% and(84.14±0.55)%, respectively, indicting that the food digestibility (%) of 4-year adults was better than that of 1-years nonages (t=6.159, df=71, P=0.000<0.05). With the temperature increase, the gross energy intake, metabolic energy and food digestibility of the different age groups of Elliot’s pheasants lowered all 12 moths of the year(Table 3, Table 4). The 29.6oC in August was the highest temperature and the metabolic energy was the smallest,while the 5.0oC of February was the lowest temperature and the metabolic energy was largest over the entire year.
Excreta, excrement energy and excrement calorific value
The excreta, excrement energy and excrementcalorific values of Elliot’s pheasant were analyzed using post hoc multiple comparisons ANOVA and paired t-Test.The results indicated there were significant differences for the excreta (4-year adults: F=72.279, P=0.000(0.05,n=72; 1-years nonages: F=23.562, P=0.000<0.05, n=72),and excrement energy (4-year adults: F=16.673, P=0.000<0.05, n=72; 1-year nonages: F=97.491, P=0.000<0.05, n=72). Of Elliot’s pheasant in the same age,the excreta was highest in autumn and lowest in summer.During the same season, the excreta of 4-year adults were smaller than 1-year nonages (t=6.517, df=71,P=0.000<0.05, Table 1, Table 2). There were no significant differences among the excrement calorific values of the 4-year adults (t=1.024, df=71, P=0.453>0.05, n=72), and nonages were also no significant differences (t=1.483, df=71, P=0.429>0.05, n=72).
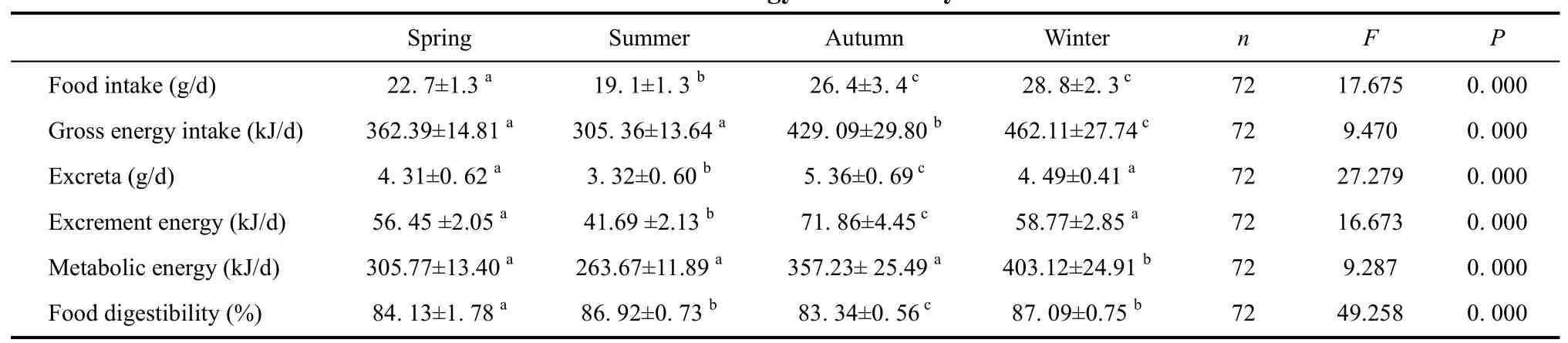
Table 1 Seasonal energy intake of 4-year adults
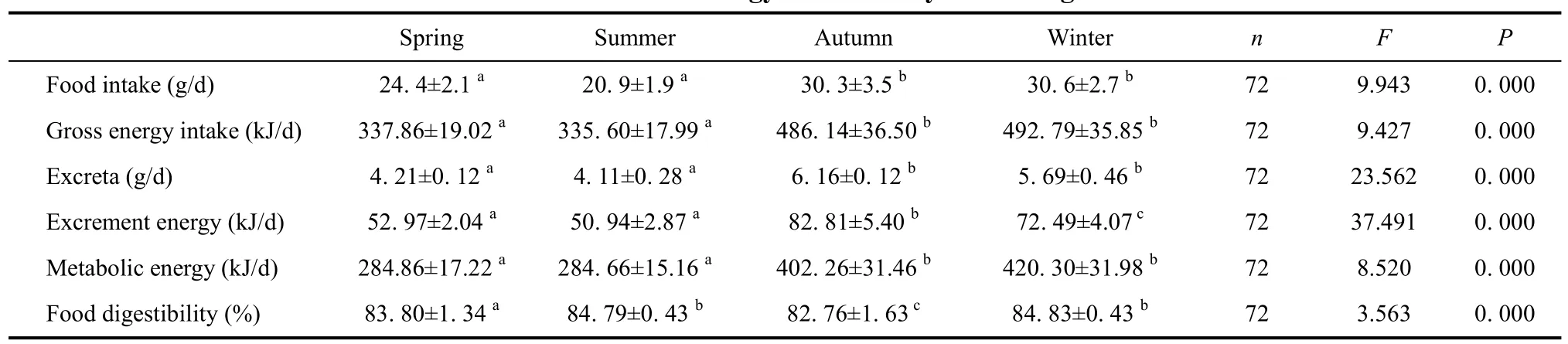
Table 2 Seasonal energy intake of 1-years nonages
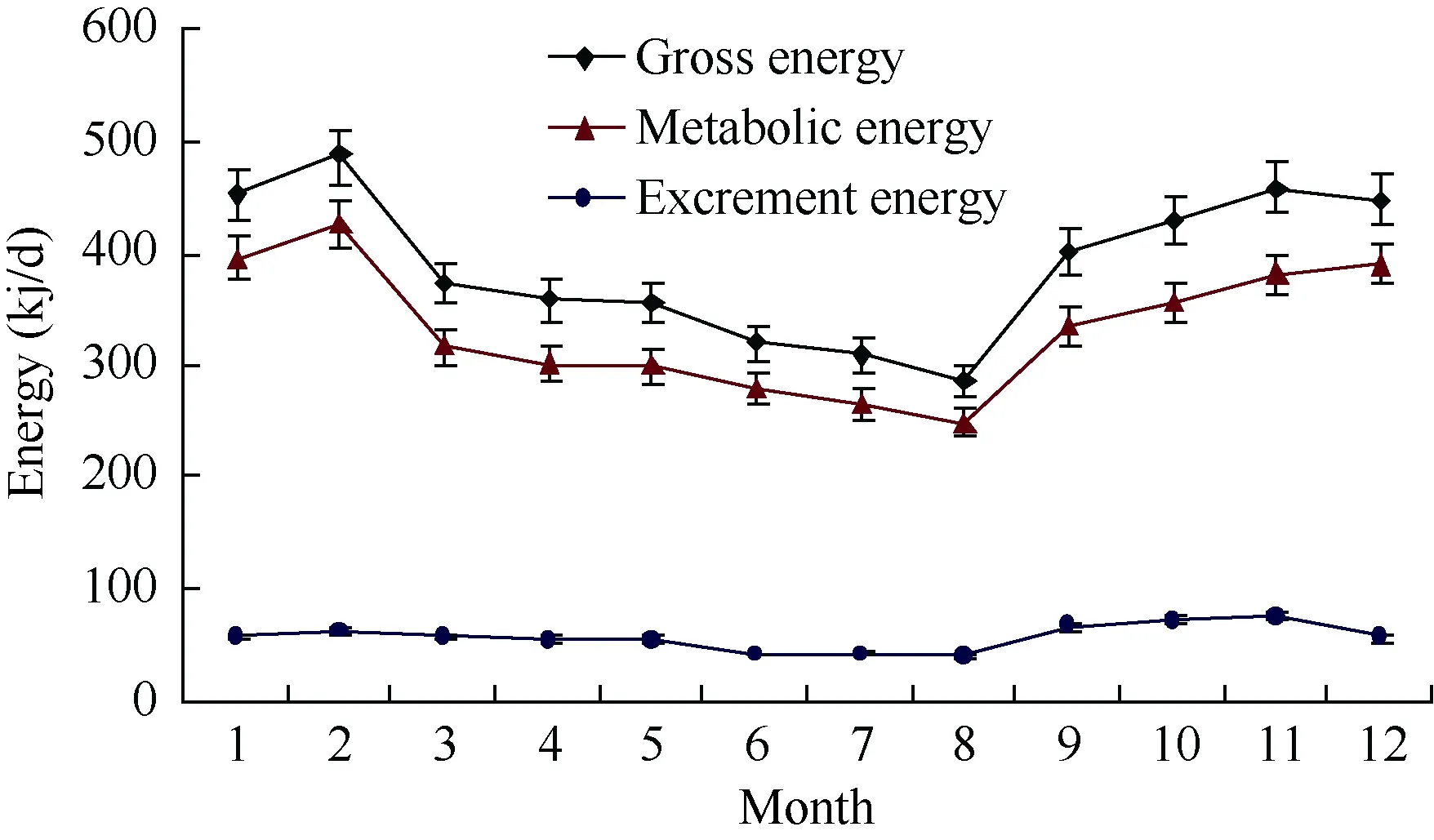
Figure 1 Monthly change in the energy intake of 4-year adults

Figure 2 Monthly change in the energy intake of 1-year nonages
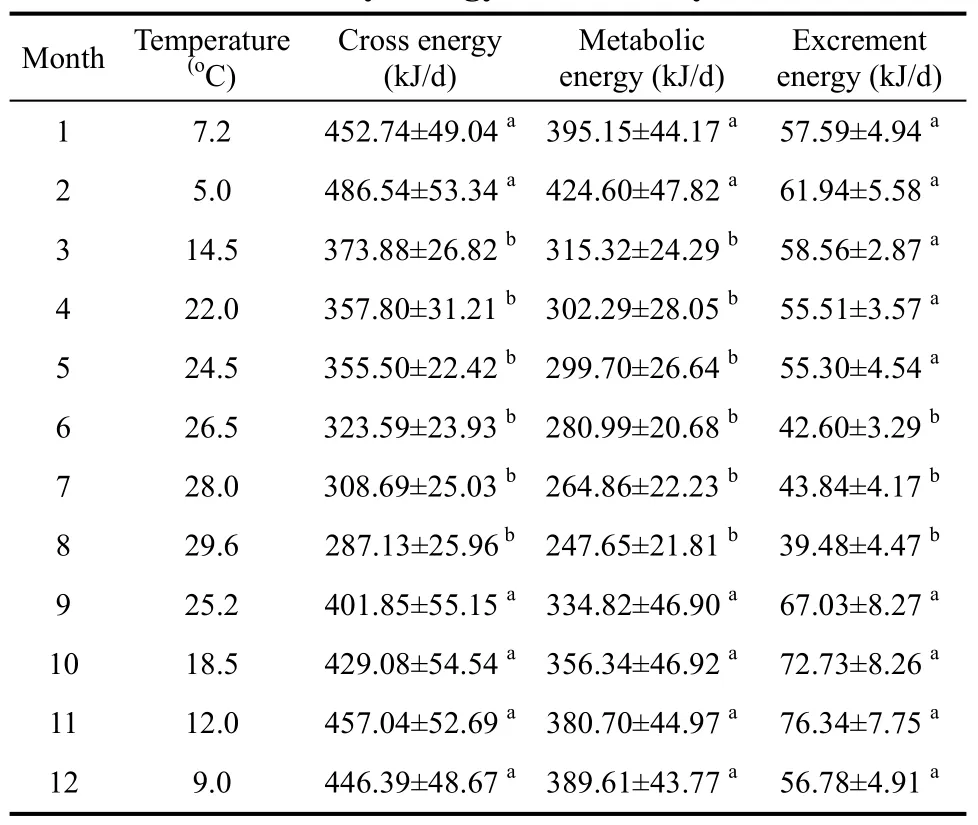
Table 3 Monthly energy intake of 4-year adults

Table 4 Monthly energy intake of 1-year nonages
Body weight
The body weight of Elliot’s pheasant nestling was analyzed by using post hoc multiple comparisons ANOVA. The results (Table 5, Figure 3) showed that the
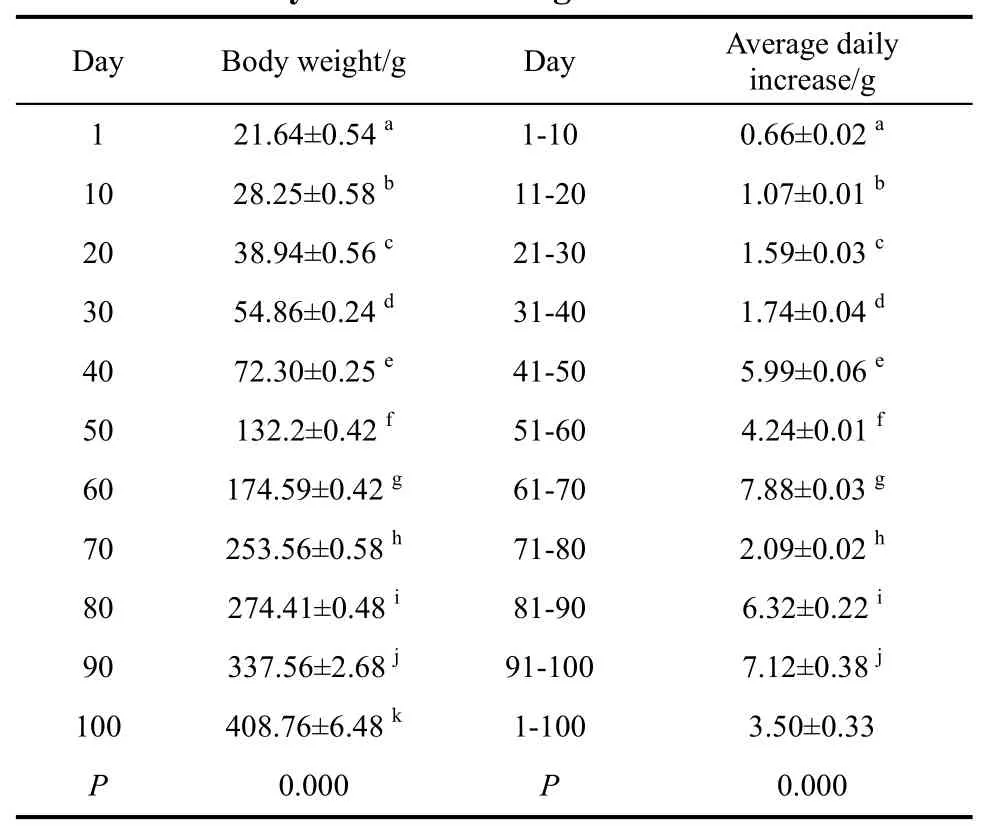
Table 5 Body weight and average daily increase of body mass of nestlings
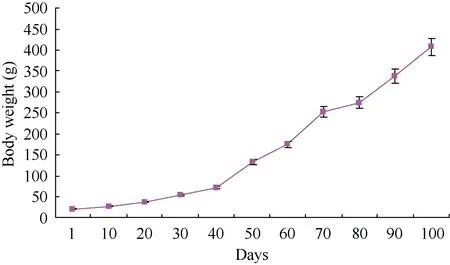
Figure 3 Daily change of nestling body weight
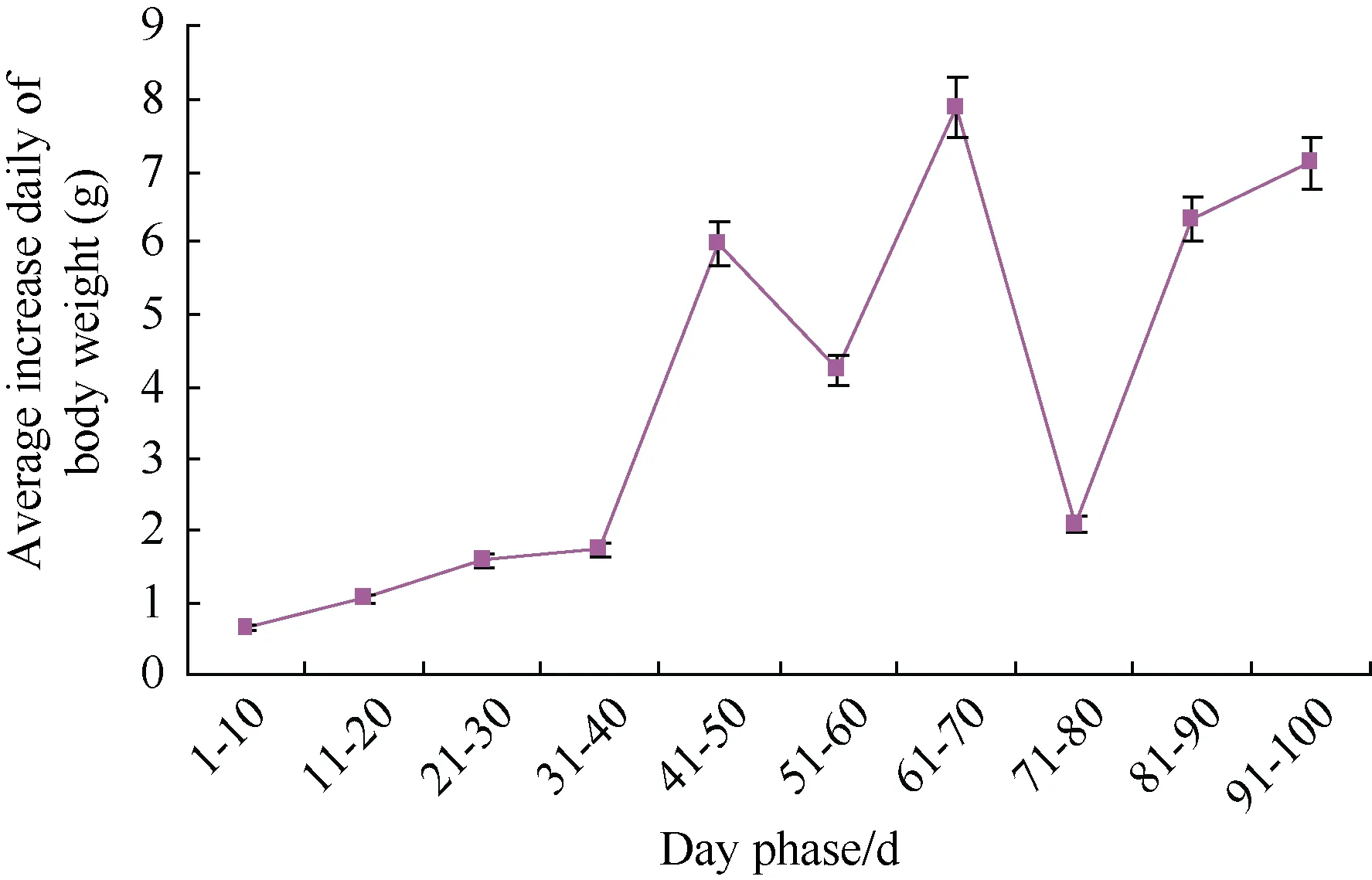
Figure 4 Average daily increase of nestling body mass
average body mass was 22.34±1.69 g at the hatching and the average daily increase of body mass was 7.88±0.03 g,which was the maximum when the nestling was 60−70 days old. The 100 day old nestling was 408.76±6.48 g,close to its adult body mass. After 100 days, the average daily increase of body weight decreased and the body weight gained slowly (Figure 4). The Syrmaticus ellioti nestling was close to 300 days when energy intake experiments were measured; each seasonal body weight had no change before and after the formal experiments:910.80±39.40 g, 925.00±53.40 g, 938.00±33.60 g and 941.20±20.20 g, for spring, summer, fall and winter respectively. Each season the body weight of 4-year adults also had no change before and after the formal experiments: 1 029.30±46.60 g, 1 017.50 ± 50.26 g,1012.40±42.50 g and 1 021.90± 30.00 g, for spring,summer, fall and winter, respectively.
DISCUSSIONS
Food and food digestibility
The analysis of energy balance of Elliot’s pheasant was established on conditions of natural light and cage situations in four different seasons. The foods afforded to the specimens were corns, compound forages and cabbages whose nutrition was very rich, and there was no significant difference for the individual body weight of birds before and after the experiment. Comparing the study results (Table 1, Table 2) with previously reported results of wild birds’ energy metabolism, the food digestibility of Elliot’s pheasant is lower than bird eating seeds. This may be one of the reasons that the foods afforded were given artificially and the study of diet selection of Elliot’s pheasant have not been previously done. A further study is needed to determine that the impact of variation wild diet of Elliot’s pheasant on its food digestibility. The energy metabolism levels of birds can also be affected by their activity (Freckleton et al,2002). As the trial pheasants exercised in a limited spatial cage, their energy metabolism level were slightly lower than wild bird eating seeds in the natural conditions, but higher than that of the Common Pheasant Phasianus cochicus (79.8%), as reported by Wang et al(2003).
Impact of environment temperature on energy intake
Energy metabolism maintains existence when the birds’ weight remains constant, thus the metabolic energy is equal to survival energy (Kendeigh, 1975). With increased temperature, the gross energy intake and metabolic energy of Elliot’s pheasant declined (Table 1,Table 2) consistent with earlier findings (Olson &Kendeigh, 1980; Zhang et al, 1998). Climate is one of the most important factors that determine individual energy consumption level of species and interspecies(Canterbury, 2002). Here, we showed that the gross energy intake, metabolic energy and excrement energy varied monthly and decreased as temperature increased.At 29.6oC August had the highest temperature and the metabolic energy was the lowest, while the 5.0oC of February was the lowest temperature and the metabolic energy was highest for the entire year. With the decreasing temperature, food digestibility decreased, and this is the reason for the increase of gross food consumption at hypothermia. To maintain the birds’energy balance as the temperature increases, the excrement energy decreased, and food digestibility is increases. The energy metabolism level of the birds directly reflects their tolerance at the low ambient temperature; the populations’ highly metabolic capacity is more adaptable to the cold environment (Likenes &Swanson, 1996; Swanson, 1995). Our study showed that the food digestibility was the highest in winter, perhaps as an adaptive response to the colder temperatures.
Analysis of excreta, excrement energy and excrement calorific value
In the same age, there were significant seasonal differences for the excreta (4-year adults: F=72.279,P=0.000<0.05, n=72; 1-years nonages: F=23.562,P=0.000<0.05, n=72) and excrement energy of Elliot’s pheasants (4-year adults: F=16.673, P=0.000<0.05,n=72; 1-year nonages: F=97.491, P=0.000<0.05, n=72).Excreta were the largest in autumn, but in summer the smallest. Altering the form of digestive tract or widen the volume of It may be an adaptive response to alter the form of digestive tract or widen the volume of it (Gross et al, 1985), and the adaptive capacity of the bowel determines the maximum rate of food digestibility(Kooyman et al, 1992) may be adaptive responses. In the same season, the excreta of 4-year adults were smaller than that of 1-year old nonages (Table 1, Table 2). This may be relative to the diversity of the structure of digestive tract. Elliot’s pheasant can tolerate the cold in winter and adapt to the environment by decreasing excreta, thereby increasing energy digestibility. There were no significant differences among the excrement calorific values of Elliot’s pheasant, but the trend of decreasing as temperature increased is in accordance with Wang’s (1996) earlier findings.
Impact of body weight and age on energy digestibility
The energy metabolism level of bird depends on their body weight (McNb, 2000). There were 2 groups of Elliot’s pheasants (4-year adults and 1-year nonages) in this experiment. The average body weight of 100 days old nestling hatched in May 2010 was 408.76±6.48 g,close to adult body weight. After 100 days, the average daily increase of body weight decreased and the body weight grew slowly (Figure 4). When nestling were 300 days old, each seasonal body mass had no change before and after the formal experiments; 910.80±39.40 g,925.00±53.40 g, 938.00±33.60 g and 941.20±20.20 g, in spring, summer, fall and winter, respectively. The average body weights of 4-year adults in spring, summer,fall and winter were 1 029.30±46.60 g, 1 021.90± 30.00 g,1 017.50±50.26 g and 1 012.40±42.50 g, respectively. In the same season, the energy digestibility of 4-year adults was stronger than 1-years nonages, as digestibility may be affected by body weight.
The Elliot’s pheasants hatched in May 2010, less than one year old, are in the growth and development phase but tend to slow as they age, and their average body weights were less than that of the 4-year old adults.The large individual animals have relatively small body surface areas, and heat loss of per unit body weight is relatively less in winter; however, in the conditions of high temperature during summer, the heat of environment flow into animal’s body is relatively small (Luo et al,2008), so the needs of gross metabolic energy of the per unit time per unit body weight of Elliot’s pheasant is less,and energy digestibility is stronger. The results indicate that the heavier birds had stronger food digestibility, in accordance with research done on the Mountain Finch Leucosticte brandti (Qian et al, 1983). We also discovered that female Elliot’s pheasant in the 4-year adults group had a higher average metabolic energy than the males per day, but their body weight was lower than the males. The reasons for this discrepancy were that the female Elliot’s pheasant of 4-year adults had to accumulate more adipose than males for reproducing in next year, and the female bird in a semi-domesticated state can maintain spawning for a relatively longer time by redistributing nutrients in their body (Zhang & Yang,2005), so the use of individual additional energy is concerned with the increase of total adipose (Wise &Weight, 1994).
From the above analysis, we can infer that there is significant difference between the gross energy intake,excrement energy, metabolic energy and temperature of different age groups of Elliot’s pheasant. At the same time, body weight and age most certainly impact food digestibility during the same season, food digestibility was higher in of 4-year adults than in 1-year nonages.Zhou (1990) reported that the food digestibility of wild birds taken seeds or nuts were (89.5%±4.7)%. Our results showed that the average food digestibility (%) of 4-year adults and juveniles were (85.44±0.54)% and(84.14±0.55)%, respectively. Elliot’s pheasant mainly eat plant leaves, stems, buds, flowers, fruits, seeds and other plant food crops; it also eats insects and other animal food (John et al, 2000). The metabolic energy of the two groups of trial pheasant were the lowest in August (29.6oC), suggesting that Elliot’s pheasant can better adapt to subtropical mountain environments through good food digestibility.
Acknowledgements: We are grateful to Professor JinSong LIU of Wenzhou University for providing valuable suggestions and references during this experiment.
- Zoological Research的其它文章
- Blockage of glucocorticoid receptors during memory acquisition,retrieval and reconsolidation prevents the expression of morphineinduced conditioned place preferences in mice
- Seed caching and cache pilferage by three rodent species in a temperate forest in the Xiaoxinganling Mountains
- Proximity association in polygynous western black crested gibbons(Nomascus concolor jingdongensis): network structure and seasonality
- 中国鸟类亚种新记录——黑冠黄鹎
- 虹鳟LECT2的酵母表达、纯化及生物活性分析
- 蛇毒抗菌肽OH-CATH对大肠杆菌引起家兔泌尿系感染的保护作用

