Synthesis and Crystal Structures and Magnetic Properties of a Nickel(Ⅱ) Complex Based on the Bipyridyl-dicarboxylic Acid
- (College of Chemistry and Environmental Engineering, Yangtze university, Jingzhou Hubei 434023)
Synthesis and Crystal Structures and Magnetic Properties of a Nickel(Ⅱ) Complex Based on the Bipyridyl-dicarboxylic Acid
LIZai-gao(College of Chemistry and Environmental Engineering, Yangtze university, Jingzhou Hubei 434023)
Treatment of hydrated NiⅡCl2with 2,2’-bipyridyl-5,5’-dicarboxylic acid (H2bpdc) in the mixed EtOH/H2O afforded a 2-D grid compounds {[NiⅡ(bpdc)(H2O)]·H2O}n(1) with 44topology. It has been characterized by IR and elemental analysis and their crystal structures have been determined by X-ray crystallography. Magnetic measurement of 1 shows that antiferromagnetic interaction occur between the paramagnetic Ni(Ⅱ) centers.
Nickel; Metal-organic complex;2,2’-bipyridyl-5,5’-dicarboxylic acid
1 Introduction
The rational design of multi-functional metal-organic frameworks (MOFs) has attracted wide attentions not only due to their intriguing topological structures but also their potential application in heterogeneous catalysis, gas sorption, storage and separation, molecular recognition, non-linear optics, ion-exchange, fluorescence and magnetism and so on[1]. One of the popular synthetic strategies is employing the suitable multi-dentate bridging ligands and transition metal ions. The pyridyl-multi-carboxylic acids are quite ligands that could satisfy such criterion and a large number of metal complexes containing these types of ligands have been constructed and their corresponding properties have been thoroughly investigated[2]. We have previously obtained a novel 3-D porous compound, {[Ni(PDC)(Cl)](Na)(H2O)/3}n, by treatment of pyridyl-2,6-dicarboxylic acid (H2PDC) and hydrated NiCl2in the presence of NaOH, which possesses a NbO-type (6482) topological network and has an uncommon nested discrete 6-1 ion-molecular [Na6(H2O)]+in its porosity[3].Recently, polytopic ligand, 2,2’-bipyridyl-5,5’-dicarboxylic acid (H2bpdc), has been proved to be an excellent ligand to construct such type materials, mainly based on the following considerations: (i) the coordination sites of N and O in this ligand are less steric; (Ⅱ) weak supra-molecular interactions such as H-bonding and π-π stacking are readily formed; (iii) the deprotonated Hbpdc-and bpdc2-are readily formed to construct various metal-organic complexes; (iv) the ligand could exhibits a wide variety of coordination modes, as shown in Figure 1. However, only few coordination polymers based on this polytopic ligand have been obtained so far. Suh et.al recently reported a linear coordination polymer, [Ni(cyclam)(bpdc)]·5H2O[4].This novel compound stacks via C-H-π interactions to form a permanent porosity and exhibits multiple functions including high porosity, hydrogen-storage capacity, and selective guest-binding ability. Other few metal-organic compounds bearing interesting structures and properties based on H2bpdc ligand have also been recently documented[5].On the other hand, coordination polymers containing paramagnetic metal ions or clusters constitute a recent topic of great interest as they have potential applications in the area of molecular magnetism such as classical multi-dimensional magnetic solids and molecular magnets[6]. Inspired by the factors above-mentioned, we intend to further exploit the versatile coordination chemistry of H2bpdc by introduction of paramagnetic metal ion Ni(Ⅱ) into the system.
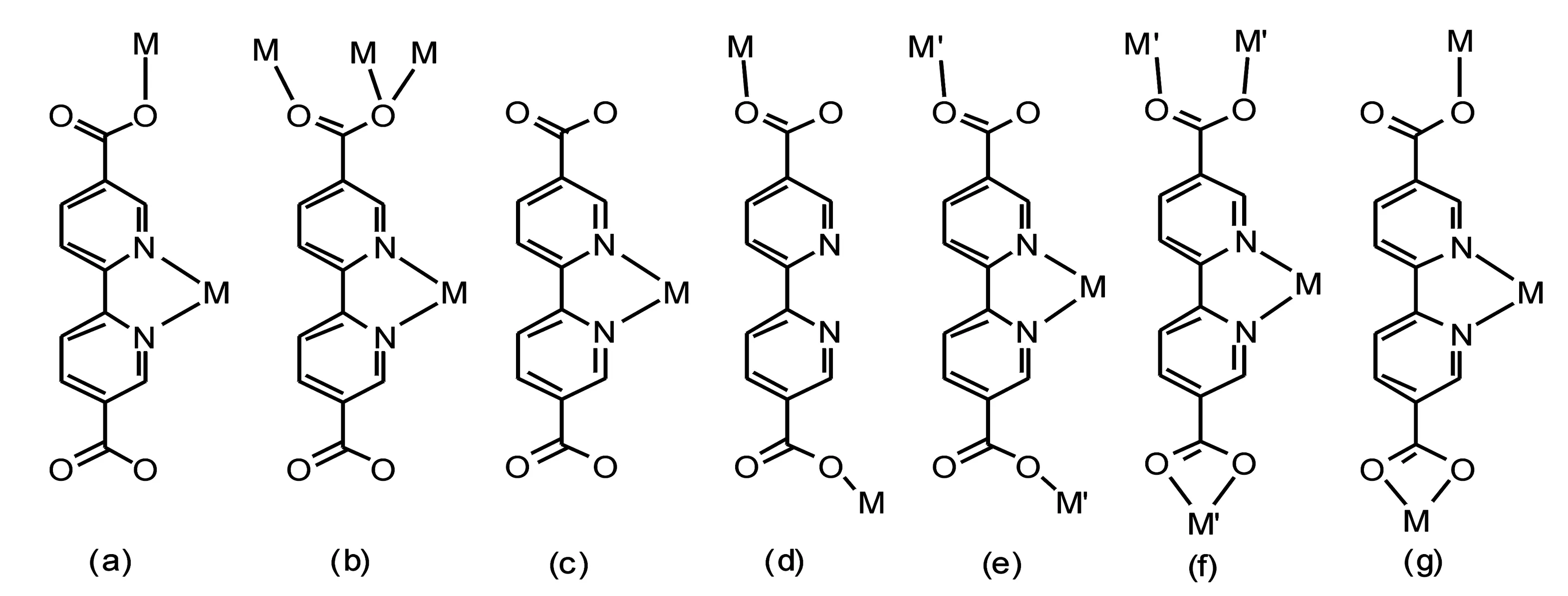
(a-f): the previously reported coordination modes; (g) the new coordination mode in this article.
2 Experimental
Preparation of 1: A mixed EtOH/H2O solution (15ml, v∶v = 1∶1) of H2bpdc (1.0mmol, 0.17g), NiCl2· 6H2O (1.0mmol, 0.24g) in a 25ml bomb was heated to 140℃ for 3 day, then cooled down to room temperature at the rate -5℃/h. Green block crystals were collected in yield of ca. 46% based on H2bpdc. Selected IR (KBr, cm-1):3315(m), 1610(s), 1433(w), 1376 (s), 1300(w), 1258(w), 1164(w), 1139(w), 1043(m), 874(m), 850(s), 774(s), 710(m). Anal. Calcd for C12H8N2O5Ni·H2O: C, 42.78, H, 2.99, N, 8.31; found: C, 42.90, H, 3.02, N, 8.27.
3 Synthesis and discussion
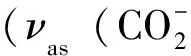
4 Conclusion
In summary, a highly thermostable metal-organic complex 1 has been solvothermally synthesized and structurally characterized. The ligand bpdc2-exhibits a new coordination mode that links three metal centers to give a 2-D irregular square with a 44topology. Variable-temperature magnetic susceptibilities indicate antiferromagnetic coupling between these paramagnetic centers. The present data further confirms that the H2bpdc is a potential useful ligand for construction of the multifunctional materials and we will introduce the other transitions metal ions or the small ancillary ligands into this system to obtain new hybrid material in future.
[1]Maspoch D, Ruiz-Molina D, Veciana J. Old materials with new tricks: multifunctional open-framework materials[J].Chem Soc Rev, 2007,36:770-818.
[2]Ke X J, Li D S, Du M.Design and construction of self-penetrating coordination frameworks[J]. Inorg Chem Commun, 2011,14:788-803.
[3]Xiang J, Yin Y G, Mei P, et al.Nested [Na6(H2O)]6+encapsulation of nickel(Ⅱ) coordination framework assembled from pyridyl-2,6-dicarboxylic acid[J]. Inorg Chem Commun, 2007,10: 455-458.
[4]Lee E Y, Suh M P.A robust porous material constructed of linear coordination polymer chains: Reversible single-crystal to single-crystal transformations upon dehydration and rehydration[J]. Angew Chem Int Ed,2004,24: 2789-2801.
[5]Kongshaug K O, Fjellvag H.Syntheses, structures and magnetic properties of Mn(Ⅱ) containing 3D polymeric networks, Polyhedron, 2007,26:5113-5119.
[6]Lu X M, Li P Z, Wang X T, et al. The 3D Channel Framework Based on Indium(III)-btec, and Its Ion-Exchange Properties (btec = 1,2,4,5-Benzenetetracarboxylate)[J]. Eur J Inorg Chem,2005, 2005:1927-1931.
[7]Tang Y Z, Wang X S, Zhou T, et al.A Novel 2D Manganese(Ⅱ) Coordination Polymer Exhibiting Ferromagnetic Interaction[J].Cryst Growth Des,2006,6:11-13.
data]2012-11-20
[Foungdationitem] Natural Science Foundation of China (21201023).
BiographyLi Zai Gao (1962-), Male, Graduted in 1984,Senior engineer,Major in Applied Chemistry.
[ChineseLibraryClassification]O634 [Documentcode]A [ArticleID]1673-1409(2013)04-N040-04

