氢键和π-π诱导的三重穿插的二维Znギ配位聚合物的晶体结构和荧光性能
胡劲松 汪茂灿 石建军 吴小雨 何 杰
(安徽理工大学化学工程学院,淮南 232001)
0 Introduction
Thedesignedconstructionof coordinationpolymers from various molecular building blocks connected by coordination bond,supramolecular contacts,has been of great interest due to their intriguing aesthetic structures and topological features[1-2],as well as their potential applications in photochemistry areas[3-4], molecular magnetism[5-6],heterogeneous catalysis[7-8],and molecular sorption[9-10].Recently,a number of multidentate carboxylate ligands have been widely employed to construct coordination polymers with fascinating architectures and interesting properties[11-12].4,4′-[(4-carboxybenzyl)nitrilo]dibenzoate (H3L),a tripod carboxylate ligand which can be regarded as a half-flexible ligand.To test the ability to obtain new architecturesand topologies,we selected this ligand,1,10-phenanthroline (Phen),and Znギsalt to solvothermally synthesize a new coordination polymers[Zn(HL)(Phen)]n(1).The compound was characterized by elemental analysis,IR spectra and X-ray crystallography.In addition,fluorescence of complex 1 was studied.
1 Experimental
1.1 Materials and methods
The reagents and solvents employed were commercially available and used as received.IR absorption spectrum of 1 was recorded in the range of 400~4 000 cm-1on a Nicolet(Impact 410)spectrometer with KBr pellet.C,H,and N analyses were carried outwith aPerkinElmer240C elemental analyzer.Luminescent spectra were recorded with a SHIMAZU VF-320 X-ray fluorescence spectrophotometer at room temperature.The as-synthesized sample was characterized by thermogravimetric analysis(TGA)on a Perkin Elmer thermogravimetric analyzer Pyris 1 TGA up to 1 023 K using a heating rate of 10 K·min-1under N2atmosphere.
1.2 Synthesis of the compound
[Zn(HL)(Phen)]n(1):A mixture of Zn(NO3)2·6H2O(15 mg),H3L(20 mg)and Phen(10 mg)was dissolved in 9 mL of DMF/H2O (2∶1,V/V).The final mixture was placed in a Parr Teflon-lined stainless steel vessel(15 mL)under autogenous pressure and heated at 90℃for 3 d.Large quantities of colorless crystals were obtained,which were washed with mother liquid,and dried under ambient conditions(Yield:63%based on H3L).Anal.Calcd.for C34H23O6N3Zn(%):C,64.31,H,3.65,N,6.62;found(%):C,64.17,H,3.91,N,6.86.IR(KBr,cm-1):3 395(w),3 052(w),1 665(m),1 603(s),1 541(s),1 373(s),1 189(m),1 012(w),851(m),790(s),721(s),652(m),582(w).
1.3 Crystal structure determination
X-ray crystallographic data of 1 was collected at room temperature using epoxy-coated crystal mounted on glass fiber.All measurements were made on a Bruker Apex Smart CCD diffractometer with graphitemonochromated Mo Kα radiation (λ=0.071 073 nm).The structure of complex 1 was solved by direct methods,and the non-hydrogen atoms were located from the trial structure and then refined anisotropically with SHELXTL using a full-matrix least-squares procedure based on F2values[13].The hydrogen atoms positions were fixed geometrically at calculated distances and allowed to ride on the parent atoms.The crystallographic data are summarized in Table 1,while the selected bond lengths and angles are given in Table 2.
CCDC:901345.
2 Results and discussion
2.1 Crystal structure
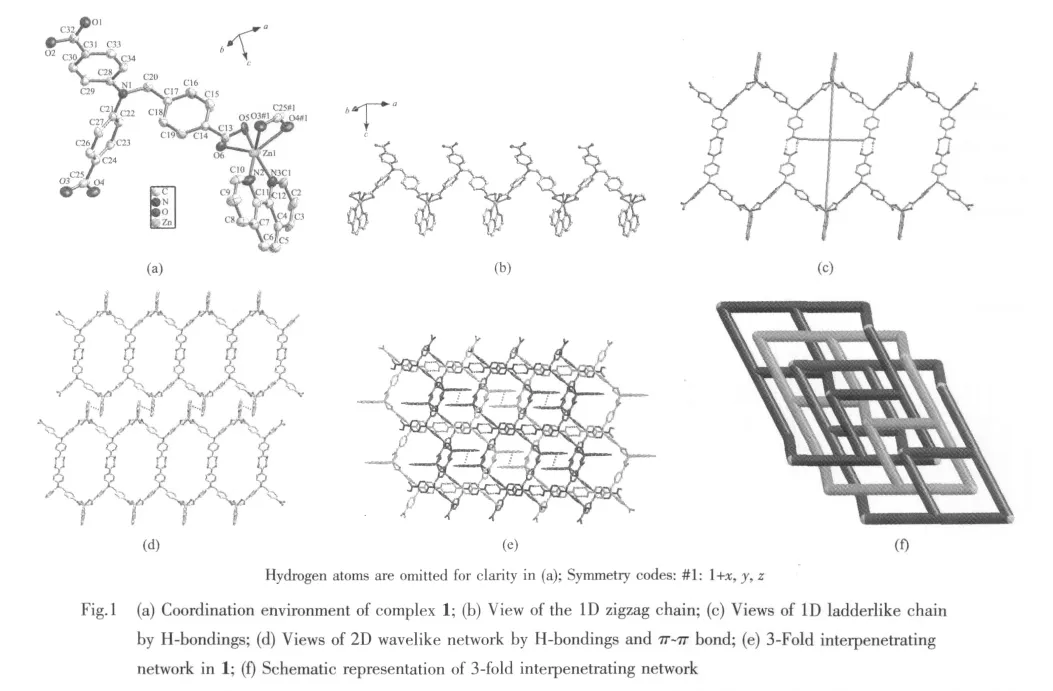
X-ray analysis reveals that compound 1 is solved in monoclinic space group P21/c.The asymmetric unit contains an independent Znギcation,one HL2-anion,one Phen ligand.The cation displays a distortedZnN2O4coordination sphere that can be best described asoctahedralgeometry.In 1,the H3L displays coordination mode with two carboxyl groups coordinated to Znギ,and the third carboxyl group is unprotoned.As shown in Fig.1a,the Znギ center is six coordinated by four carboxylic O atoms from two H2L2-ligands,two N atoms from Phen ligand.The Zn-N lengths are 0.210 5 and 0.207 3 nm,and the Zn-O lengths are in the range of 0.201 9(2)~0.228 4 nm.The dihedral angles between the three phenyl rings in the anion are 54.799(1)°,82.211(9)°and 87.635(1)°.
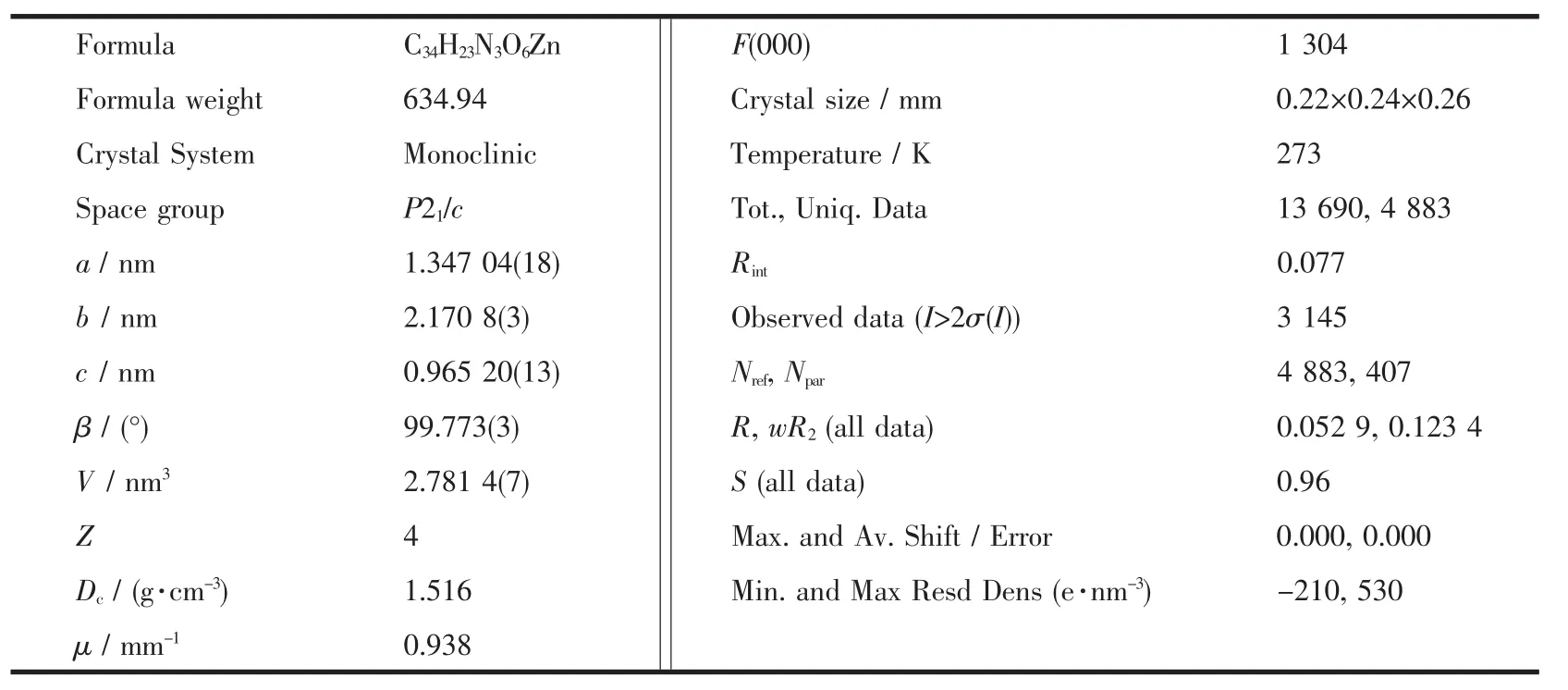
Table 1 Crystal data and structure refinement for complex 1
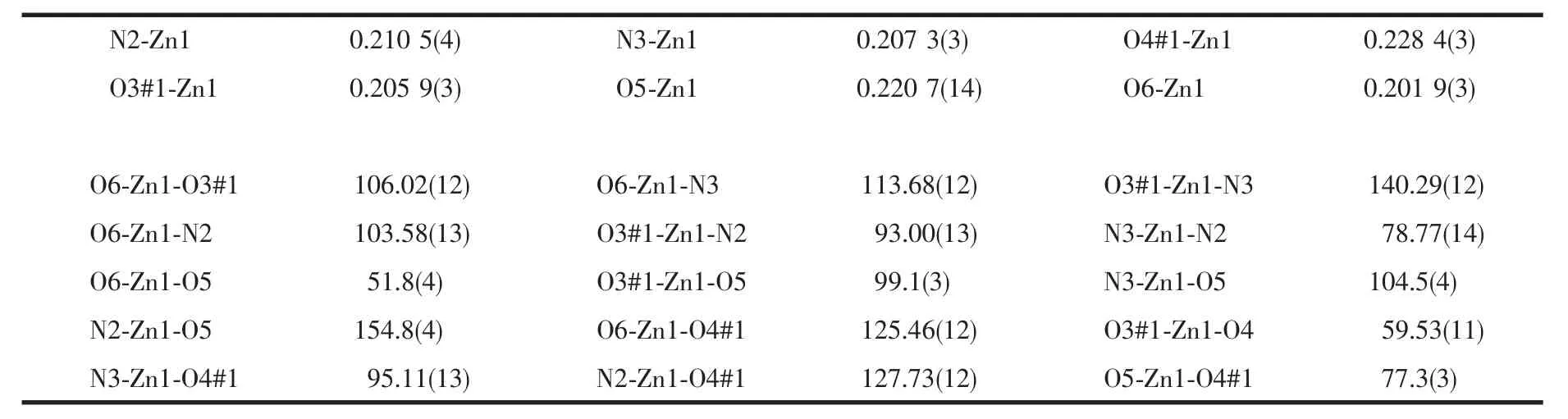
Table 2 Selected bond lengths(nm)and angles(°)for the complex 1
The neighboring Znギions are linked by HL2-anions and Phen to form infinitely 1D zigzag chain,the adjacent distance of Zn…Zn is 1.347 0 nm(Fig.1b).Further inspection shows that the carboxyl groups of every two identical chains are actually contacting each other to form 1D ladderlike chain containing hexagon grids by strong H-bonding(d(D…A)=0.2658 nm,d(D…H)=0.085 nm,d(H…A)=0.182 nm,∠(DHA)=168.4°),the size is ~1.15 nm ×2.33 nm(Fig.1c).These ladderlike chains further linked each other by π-π interactions to generate two dimensional wavelike sheets(Fig.1d).The most striking feature of complex 1 is that three identical 2D single nets interlocked each other to form 3-fold interpenetrated network.As shown in Fig.1e,the channels of each network are threaded by two pairs of interactional phen ligands from other two sheets(Fig.1f).
2.2 Thermal analysis
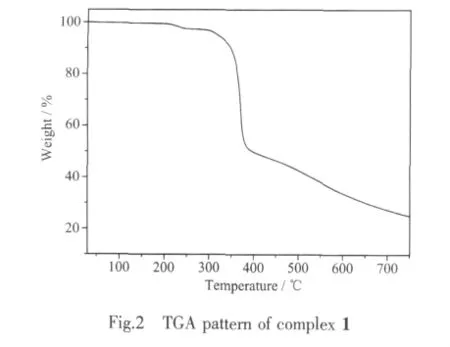
To characterize the complex more fully in terms of thermal stability,the thermal behavior of 1 was studied byTGA.Therewasnosolventin the structure,and it decomposed starting at 330℃.
2.3 Fluorescence properties
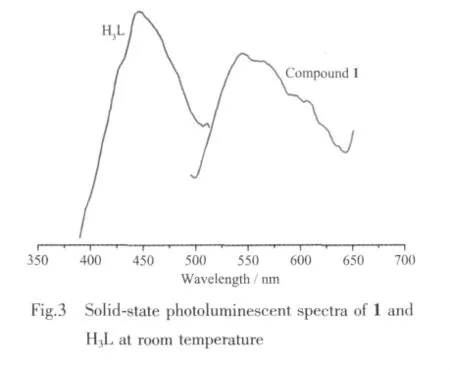
The fluorescence properties of free ligands Phen,H3L,and complex 1 in the solid state at room temperature are investigated.Intense emissions of the freeH3L and Phen ligandswereobserved with wavelength from 380 to 550 nm (λmax=440 and 380 nm),which could be attributed to the π*→π or π*→n transitions.Complex 1 exhibits mission characteristics similar to the free ligands.The emission peaks at 545 nm (λex=400 nm)shows a highly red shift compared with that of the H3L and Phen ligands,which is assigned to the chelation of the carboxylate and Phen to the central metals and the increase of conjugation upon metal coordination.
3 Conclusions
In summary,a new complex[Zn(HL)(Phen)]nhas been synthesized under solvothermal conditions.Complex 1 is an infinitely 1D chain,which further to generate 3-fold interpenetrated 2D framework by H-bonded and π-π interactions.The results demonstrate that the non-coordinated carboxyl acids can be well used as the structure-directing tool and can produce various H-bonding in the synthesis ofunusual coordination frameworks.Subsequent works will be focused on the structures and properties of a series of coordination polymers constructed by H3L with more auxiliary ligands and metal ions.
[1]Ma Y,Cheng A L,Gao E Q.Cryst.Growth Des.,2010,10(7):2832-2834
[2]Fu J H,Li H J,Mu Y J,et al.Chem.Commun.,2011,47(18):5271-5273
[3]FU Jun-Dan(傅君丹),YE Li-Qing(叶莉青),ZHANG Chun-Yan(张春艳),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(1):179-183
[4]Feng R,Jiang F L,Chen L,et al.Chem.Commun.,2009,45:5296-5298
[5]Bi Y F,Wang X T,Wang B W,et al.Dalton Trans.,2009,12:2250-2254
[6]Sarma R,Deka H,Boudalis A K,et al.Cryst.Growth Des.,2011,11:547-554
[7]Corma A,García H,Llabrés i Xamena F X.Chem.Rev.,2010,110(8):4606-4655
[8]Huang Y B,Liu T F,Lin J X,et al.Inorg.Chem.,2008,50(15):2191-2198
[9]Lin J B,Zhang J P,Chen X M.J.Am.Chem.Soc.,2010,132(19):6654-6656
[10]Chen S S,Chen M,Takamizawa S,et al.Chem.Commun.,2011,47(17):4902-4904
[11]Lan Y Q,Li S L,Shao K Z,et al.Cryst.Growth Des.,2010,10(8):3490-3492
[12]TAO Wu(陶武),LIU Jie-Mi(刘杰民),ZHENG Yan-Jun(郑延军),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(12):2419-2424
[13]Bruker 2000,SMART(Version 5.0),SAINT-plus(Version 6),SHELXTL(Version 6.1),and SADABS(Version 2.03),Bruker AXS Inc.,Madison,WI.