Analysis of 30 patients with persistent or recurrent squamous cell carcinoma of the cervix within one year after concurrent chemoradiotherapy
Shi-Ping Liu, Jia-Xin Yang, Dong-Yan Cao, Keng Shen
Department of Obstetrics and Gynecology, Peking Union Medical College Hospital, Peking Union College, Chinese Academy of Medical Science, Beijing 100730, China
Introduction
Concurrent chemoradiotherapy (CCRT) is the main and effective treatment means for advanced cervical cancer.However,there are still a number of patients developing persistent or recurrent disease after definitive CCRT.Once this occurs, the prognosis is very poor.This paper retrospectively analyzed the recurrence sites, risk factors and prognosis of 30 patients with persistent or recurrent squamous cell carcinoma (SCC) of the cervix within 1 year after CCRT, aiming at providing some guidance for the clinical practice.
Materials and methods
Clinical data
A total of 30 patients with persistent or recurrent SCC of the cervix within 1 year after undergoing the initial chemoradiotherapy at Peking Union Medical College Hospital were assigned to the recurrence group (age range: 29-66 years; median age: 45.5 years).These patients underwent chemoradiotherapy between July 2006 and July 2011 and complete clinical data were obtained from that period.A persistent lesion, or a new lesion which occurred within 3 months after the radiotherapy, was taken as the index for persistent disease, and the new tumor occurring after complete remission accompanied by progressively elevated serum SCCAg levels was taken as the recurrence index.A total of 35 SCC patients were randomly selected from those who were concurrently treated in the same hospital and showed no signs of recurrence, as confirmed by physical examination, imaging,and serum SCCAg levels after more than 1-year follow-up.These 35 patients were assigned to the control group (age range:34-66 years; median age: 47 years).
Methods
Clinical characteristics including age, FIGO stage, tumor size,lymph node status, serum SCCAg level before radiotherapy,radiotherapy time, radiotherapy dose, and cycle of concurrent chemotherapy were analyzed.Pre-radiotherapy lymph node status was assessed according to the imaging results (abdominal and pelvic CT, MRI or PET-CT).Pelvic and/or para-aortic lymphadenectasis detected by the imaging examination were deemed as lymph node metastasis.Serum SCCAg level of all patients was detected within 2 weeks before the start of the radiotherapy.The tumor differentiation level was not included in the statistical analysis since some pathology reports failed to provide it.Upon the end of radiotherapy, the follow-up was started.The following time points marked the end of follow-up:July 31, 2012, when patients died, or loss to follow-up.The study was approved by the institutional ethics committee.Written informed consent was obtained from all patients.
Statistical analysis
All data were processed with SPSS 19.0, and statistical analyses of categorical variables were performed using Chi-square test or Fisher exact test.Logistic regression was adopted for multivariate analysis, and statistical significance was defined as P<0.05.Kaplan-Meier method was employed for survival analysis.
Results
Radiotherapy
All of the patients were treated with pelvic radiation and vaginal brachytherapy.For patients with suspected para-aortic lymph node metastasis, extended-field pelvic and para-aortic radiotherapy was performed.The radiotherapy durations of 4 patients from each group were longer than 56 d, and the average total point A dose was 89.4 and 87.5 Gy, respectively.The differences in both the radiotherapy duration and dose were not statistically significant(P>0.05).During the radiotherapy, all patients received concurrent cisplatin chemotherapy (40 mg/m2, once a week).The average cycle of concurrent chemotherapy for each group was 4.4 and 4.7,respectively, with no significant difference (P>0.05).
Recurrence sites
Among the 30 patients in the recurrence group, distant metastasis occurred in 25 patients (83.3%), and recurrence sites of 5 patients (16.7%) were confined to the pelvic cavity.Of the 25 patients with distant metastasis, 21 patients (70.0%)developed only distant metastasis without pelvic failure or recurrence (Table 1).Notably, 14 patients suffered from distant metastasis within 6 months after undergoing radiotherapy.

Table 1 Sites of recurrence, n (%)
Risk factors
Univariate analysis demonstrated that the proportion of the patients with pre-therapeutic pelvic and/or para-aortic lymphadenectasis and SCCAg >10 ng/mL was higher in the recurrence group than in the control group (P<0.05, Table 2).Multivariate analysis by logistic regression also showed that the pre-chemoradiotherapy lymphadenectasis and SCCAg >10 ng/mL were the independent risk factors for persistent or recurrent SCC of the cervix within 1 year after radiotherapy.
Prognosis
In the recurrence group, 4 patients were lost to follow-up.Among the remaining 26 patients, only 1 patient with local recurrence limited to the cervix was completely relieved from thedisease after 6 courses of chemotherapy, and showed no signs of recurrence during the 31-month follow-up.Two patients received 12 and 13 interrupted chemotherapy courses, respectively,during which the disease was partially relieved or stabilized, and the follow-up for both patients lasted for 3 years.Meanwhile, 19 patients suffered from disease progression or could not stand the chemotherapy after undergoing an average of 3.3 cycles of chemotherapy, and were thus given symptomatic and supportive treatment.Two patients received symptomatic and supportive treatment after palliative radiotherapy, and 2 patients received simply the symptomatic and supportive treatment due to poor general conditions.The 2-year survival rate of the patients with persistent or recurrent disease was 21.7%, with median survival period of 17 months (Figure 1).

Table 2 Univariate analysis of potential risk factors for patients with persistent or recurrent disease, n (%)
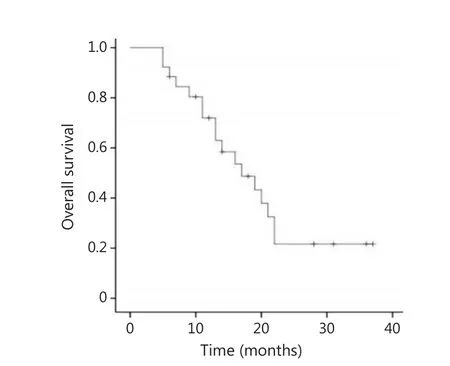
Figure 1 Overall survival of patients with persistent or recurrent disease.
Discussion
Since CCR was clinically used, the survival rate of patients with advanced cervical cancer has greatly increased in recent years.However, many patients still suffer from persistent or recurrent disease after CCR.Eifel et al.1reported that the local and distant recurrence rate of locally advanced cervical cancer after CCR was 17% and 18%, respectively.The prognosis of such patients was very poor.Hong et al.2reported that the 5-year survival rate of patients with distant metastasis or local recurrence after radiotherapy was about 10%.Furthermore, the shorter the interval between the end of the therapy and the recurrence was,the poorer the prognosis was3-5.In the current study, the 2-year survival rate of the patients with persistent or recurrent SCC of the cervix within 1 year after CCR was 21.7%, and the median survival period of these patients was 17 months.
In recent years, some scholars have studied the risk factors for radiotherapy failure of cervical cancer.Hirakawa et al.6summarized 108 patients with SCC of the cervix, and found that elevated SCCAg at the end of the radiotherapy, pre-radiotherapy anemia and lymph node metastasis were associated with distant metastasis.They also identified lymph node metastasis as the risk factor for intrapelvic recurrence.Hong et al.2reported that young age and late-stage disease were associated with local recurrence,while late-stage disease, SCCAg >10 ng/mL, and pelvic lymph node metastasis were the independent risk factors for distant metastasis.Zhang et al.7conducted a retrospective analysis of 213 patients, and found that tumor diameter ≥4 cm and tumor pathological grade 3 were the risk factors for local failure or recurrence after radiotherapy.Yu et al.8reported that late FIGO stage, tumor size, and cycle of concurrent chemotherapy influenced local control of advanced cervical cancer.Song et al.9reported that the radiotherapy time >56 days was detrimental to pelvic control in cervical cancer patients undergoing CCR;however, the lengthening of the radiotherapy time did not increase the distant metastasis rate of the patients.Huang et al.10reported that the pre-treatment serum carcinoembryonic antigen (CEA) ≥10 ng/mL was the risk factor for para-aortic lymph node recurrence following de finitive CCR for SCC of the uterine cervix.Kang et al.11, meanwhile, established a model for predicting the distant metastasis risk of locally advanced cervical cancer after CCR (pelvic and para-aortic nodal positivity on FDG-PET, non-squamous cell histology, and pretreatment serum SCC antigen levels).This model was of high resolution and calibration level.In addition, several reports12showed that the mean apparent diffusion coefficient value on diffusion weighted magnetic resonance imaging can be used to identify patients with a risk of disease recurrence.To the best of our knowledge, however, there have been no reports specially describing the risk factors for persistent or recurrent cervical carcinomas within a short period after CCR.According to the preliminary results of the current study, pre-radiotherapy pelvic and/or para-aortic lymphadenectasis and SCCAg >10 ng/mL were the risk factors for persistent or recurrent SCC of the cervix within a short period after radiotherapy.This result is consistent with the results of the above-mentioned studies to some extent,since over 80% of the recurrences occurred within 2 years after radiotherapy.However, our data showed that the distant metastasis occurred earlier and indicated a high probability.Among the 21 patients with only distant metastasis, 14 patients developed distant metastasis within 6 months after radiotherapy,which was much shorter than the distant metastasis occurrence time reported by other studies2(the median time was about 18 months).Given this, the possibility that these patients might have micrometastasis before radiotherapy could not be excluded.Treatment options for patients with persistent or recurrent disease are various, including chemotherapy, radiotherapy,surgery and palliative treatment and the specific conditions of the patients play a key role in the treatment choice.In this study, the specific conditions of our patients, such as high distant metastasis rate (83.3%), persistent disease or recurrence within a short period after radiotherapy, and so on, indicated that the following treatment consisted mainly of systematic chemotherapy.At present, the cisplatin-based chemotherapy is used as the standard treatment option for recurrent cervical cancer, but its effect is not good enough13-16.One study17showed that the supportive care-based therapy was potentially more cost-effective than the current standard doublet chemotherapy for patients with recurrent cervical cancer.
In order to improve the prognosis of such patients, sufficient and timely primary CCR is warranted.Besides, the evaluation and treatment strategies should be further improved.As described above, patients who developed distant metastasis within a short period after radiotherapy may suffer from neglected metastasis.Thus, it is necessary to undertake a comprehensive assessment of the patients with such high risk factors as pelvic and/or para-aortic lymph node metastasis and markedly elevated SCCAg before radiotherapy, in order to discover the micrometastasis and develop a rational treatment plan.Some reports13,18-20showed that chemotherapy (including neoadjuvant chemotherapy and consolidation chemotherapy)can improve the prognosis of the cervical carcinoma patient affected by unfavorable prognosis factors.Hong et al.2reported that the prognosis of the patients with recurrent disease limited to para-aortic lymph node was better than that of patients with supra-clavicular lymph node metastasis.Furthermore, they reported that the prognosis of patients with recurrent disease restrained to the cervix was better than that of patients with recurrent disease beyond the cervix.Thus, intense follow-up of patients with a high risk for recurrence is required in order to discover earlier recurrence and make timely remedy measures.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Eifel PJ, Winter K, Morris M, Levenback C, Grigsby PW, Cooper J, et al.Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervicalcancer: an update of radiation therapy oncology group trial (RTOG) 90-01.J Clin Oncol 2004;22:872-880.
2.Hong JH, Tsai CS, Lai CH, Chang TC, Wang CC, Chou HH, et al.Recurrent squamous cell carcinoma of cervix after de finitive radiotherapy.Int J Radiat Oncol Biol Phys 2004;60:249-257.
3.Eifel PJ, Jhingran A, Brown J, Levenback C, Thames H.Time course and outcome of central recurrence after radiation therapy for carcinoma of the cervix.Int J Gynecol Cancer 2006;16:1106-1111.
4.Moore DH, Tian C, Monk BJ, Long HJ, Omura GA, Bloss JD.Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study.Gynecol Oncol 2010;116:44-49.
5.Zanetta G, Torri W, Bocciolone L, Lucchini V, Mangioni C.Factors predicting response to chemotherapy and survival in patients with metastatic or recurrent squamous cell cervical carcinoma: a multivariate analysis.Gynecol Oncol 1995;58:58-63.
6.Hirakawa M, Nagai Y, Inamine M, Kamiyama K, Ogawa K, Toita T, et al.Predictive factor of distant recurrence in locally advanced squamous cell carcinoma of the cervix treated with concurrent chemoradiotherapy.Gynecol Oncol 2008;108:126-129.
7.Zhang M, Cai S, Wang X.Analysis of patients with persistent cervical cancer after radiotherapy.Zhonghua Fu Chan Ke Za Zhi 1999;34:692-693 (in Chinese).
8.Yu L, Lou G, Zhang Y.Analysis of factors related with local failure of advanced cervical cancer after concurrent chemoradiotherapy.Shiyong Fu Chan Ke Za Zhi 2006;22:359-361 (in Chinese).
9.Song S, Rudra S, Hasselle MD, Dorn PL, Mell LK, Mundt AJ,et al.The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy.Cancer 2013;119:325-331.
10.Huang EY, Huang YJ, Chanchien CC, Lin H, Wang CJ, Sun LM, et al.Pretreatment carcino embryonic antigen level is a risk factor for para-aortic lymph node recurrence in addition to squamous cell carcinoma antigen following de finitive concurrent chemoradiotherapy for squamous cell carcinoma of the uterine cervix.Radiat Oncol 2012;7:13.
11.Kang S, Nam BH, Park JY, Seo SS, Ryu SY, Kim JW, et al.Risk assessment tool for distant recurrence after platinum-based concurrent chemoradiation in patients withlocally advanced cervical cancer: a Korean gynecologic oncology group study.J Clin Oncol 2012;30:2369-2374.
12.Nakamura K, Joja I, Nagasaka T, Fukushima C, Kusumoto T, Seki N, et al.The mean apparent diffusion coefficient value (ADCmean)on primary cervical cancer is a predictive marker fordisease recurrence.Gynecol Oncol 2012;127:478-483.
13.Tang J, Tang Y, Yang J, Huang S.Chemoradiation and adjuvant chemotherapy in advanced cervical adenocarcinoma.Gynecol Oncol 2012;125:297-302.
14.Leath CA 3rd, Straughn JM Jr.Chemotherapy for advanced and recurrent cervical carcinoma: results from cooperative group trials.Gynecol Oncol 2013;129:251-257.
15.Pectasides D, Kamposioras K, Papaxoinis G, Pectasides E.Chemotherapy for recurrent cervical cancer.Cancer Treat Rev 2008;34:603-613.
16.Kitagawa R, Katsumata N, Shibata T, Nakanishi T, Nishimura S,Ushijima K, et al.A randomized, phase III trial of paclitaxel plus carboplatin (TC) versus paclitaxel plus cisplatin (TP) in stage IVb,persistent or recurrent cervical cancer: Japan Clinical Oncology Group study (JCOG 0505).J Clin Oncol 2012;30:328.
17.Phippen NT, Leath CA 3rd, Miller CR, Lowery WJ, Havrilesky LJ, Barnett JC.Are supportive care-based treatment strategies preferable to standard chemotherapy in recurrent cervical cancer?Gynecol Oncol 2013;130:317-322.
18.Choi CH, Lee YY, Kim MK, Kim TJ, Lee JW, Nam HR, et al.A matched-case comparison to explore the role of consolidation chemotherapy after concurrent chemoradiationin cervical cancer.Int J Radiat Oncol Biol Phys 2011;81:1252-1257.
19.Singh RB, Chander S, Mohanti BK, Pathy S, Kumar S, Bhatla N, et al.Neoadjuvant chemotherapy with weekly paclitaxel and carboplatin followed by chemoradiation in locally advanced cervical carcinoma: a pilot study.Gynecol Oncol 2013;129:124-128.
20.Abe A, Furumoto H, Nishimura M, Irahara M, Ikushima H.Adjuvant chemotherapy following concurrent chemoradiotherapy for uterine cervical cancer with lymphadenopathy.Oncol Lett2012;3:571-576.
 Cancer Biology & Medicine2013年4期
Cancer Biology & Medicine2013年4期
- Cancer Biology & Medicine的其它文章
- Asian trends in primary androgen depletion therapy on prostate cancer
- Therapeutic resistance in cancer: microRNA regulation of EGFR signaling networks
- Why bortezomib cannot go with ‘green’?
- Effects of HLEC on the secreted proteins of epithelial ovarian cancer cells prone to metastasize to lymph nodes
- Rare myeloid sarcoma/acute myeloid leukemia with adrenal mass after allogeneic mobilization peripheral blood stem cell transplantation
- Instructions for Authors
