Asian trends in primary androgen depletion therapy on prostate cancer
Hideyuki Akaza
Department of Strategic Investigation on Comprehensive Cancer Network, Research Center for Advanced Science and Technology, The University of Tokyo, Tokyo 113-8654, Japan
Introduction
It is well known that there are significant regional differences in the incidence and mortality rates of prostate cancer.The difference is particularly notable between the Asian region and European countries.Prostate cancer is an androgen sensitive cancer and responds well to androgen depletion therapy (ADT),however, one of the unique drawbacks to this treatment is that it causes testosterone losing syndrome.It is for this reason that in Western countries the role of ADT in treating prostate cancer is generally limited to metastatic cancer and incurable advancedstage cancer.On the other hand, in many of the countries in Asia,including Japan, ADT has for many years been relatively often used also for localized cancer, which perhaps re flects the social and philosophical background of Asia.
However, comparative studies of the outcomes of ADT in Asia and the West have actually only recently been initiated,and the clinical significance of ADT in both Asia and the West remains unclear.
In this paper the history of a unique joint collaborative study in Asia on ADT will be introduced and an overview of a registry study, which has developed out of previous efforts, will also be introduced.The significance of ADT for prostate cancer treatment will be discussed.
The history of Asian collaborative study of ADT on prostate cancer (Table 1)
An international conference titled “Asian trends in prostate cancer hormone therapy” was first held in 2001, with committee members comprising primarily urology specialists from a number of regions, including Japan, China, Korea, Singapore,Indonesia and Taiwan.The first conference in 2001 was held in Singapore, followed by the second in Hong Kong (2002),third in Tokyo (2003), fourth in Honolulu (2004), and the fifth in Bali, Indonesia (2006)1-4.At the fifth conference 27 urooncologists from Asia participated.This conference was also attended by Dr.Malcolm Moore, inaugural director of the Union for International Cancer Control (UICC) Asia Regional Office(ARO), who emphasized the necessity for the construction of a registration system and the importance of screening, prevention and diet control5.
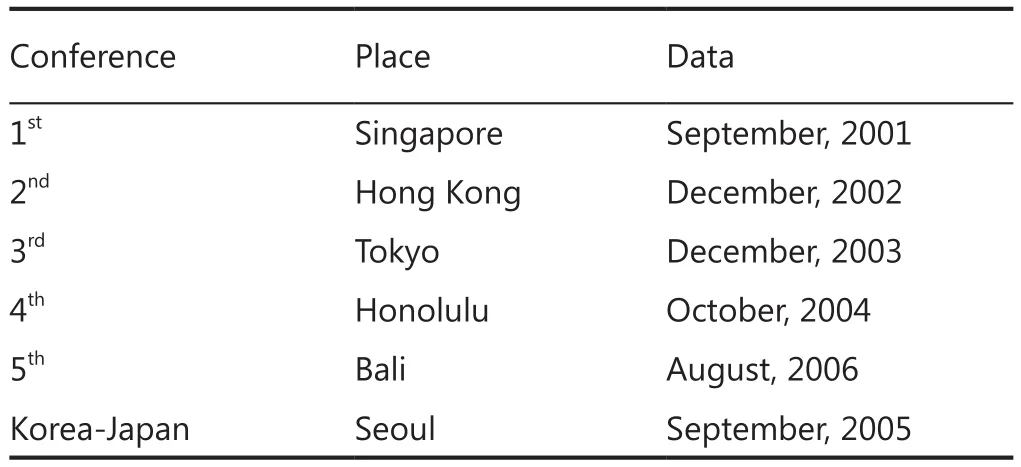
Table 1 Conference of Asian trends in prostate cancer hormone therapy
Similar meetings were also held in 2005 on a bilateral basis between Japan and Korea.These various approaches were discussed and brought together at a Prostate Cancer Working Group meeting held at the 20thAsia-Pacific Cancer Conference(APCC) in Tsukuba, Japan in 20106.
Through the course of these various meetings, a wideranging discussion took place on the current status of ADT for prostate cancer treatment in Asia and the various issues being faced overall.At the first meeting data was collected concerning the patient characteristics of prostate cancer patients in Asia,including the proportion that was administered ADT at various stages of cancer.Although the scale of data sources differed from country to country, the proportion of patients being administered ADT as an initial therapy at each cancer stage was compared, and it was shown that at each cancer stage ADT was selected as a therapy for a relatively large proportion of patients1.At the second meeting, in an attempt to uniformalize data sources, 100 recently diagnosed new patients were registered from among participating member institutions and a comparison was implemented.In the comparison of the cancer stage of each patient at which ADT was administered as an initial therapy, with the exception of Singapore, all other countries reported that there were many cases in which ADT was selected for localized prostate cancer.In the case of Singapore, it was mainly T4patients who received ADT2.At the third meeting a comparison of quality of life was implemented,using the same method as before, with 100 registered patients from the various countries.The same questionnaire was issued to patients for comparison purposes.Furthermore, at the third meeting discussion took place concerning the number of prostate biopsy cores and also on the need for and feasibility of implementing a joint multi-institutional study in Asia3.In the Prostate Cancer Working Group that was organized as part of the APCC, discussion took place concerning the low incidence of prostate cancer in Asia and the relationship between this low incidence and the large consumption of soy-based food products.In addition, discussion also focused on a comparison between Asia and the West with regard to the significance of ADT and on the necessity to establish and interpret unique guidelines for ADT in Asia.The conference on Asian trends in prostate cancer hormone therapy was ultimately disbanded due to a lack of operating funds.However, the spirit of that conference remains with us today, in the form of the Asia-Pacific Prostate Society7.
Asian consensus statement for NCCN clinical practice guidelines of prostate cancer
The National Comprehensive Cancer Network (NCCN) clinical practice guidelines are used widely and frequently in all regions of the world, not just in the cancer center in the United States where they originated.They have become the standard for cancer treatment.However, given the various conditions in each country and region, it does not necessarily mean that all medical institutions can provide treatment that is exactly in accordance with the guidelines.In particular, various factors in Asia,including those of a social, economic, philosophical, medical and religious nature, are obstructing implementation in accordance with the guidelines.The compilation of any guideline should be implemented on the basis of medical evidence, using cohort studies, and the situation in Asia is that there is still a lack of such medical evidence, meaning that the only option is to utilize the global guidelines as set forth by the NCCN.
The Asian consensus statement (ACS) for NCCN clinical practice guidelines takes these various constraints into consideration and represents the results of discussion about how the countries of Asia can implement treatment that matches the conditions in each particular country.In the field of urological malignant tumors, the ACS is being followed in the two areas of renal cancer and prostate cancer8,9.
From these efforts it has become apparent that the method of use of endocrine therapy for prostate cancer is different in Asia compared to the West.In other words, in Asia, even in cases where prostate cancer has been diagnosed at a relatively early stage, the use of primary androgen depletion therapy (PADT) is being promoted.In September 2013 ACS committee meeting for NCCN clinical practice guideline for prostate cancer was held in Inchon, Korea under the auspice of Asia Pacific Prostate Society,Asia Pacific Society of Uro-oncology and Japanese Society of Clinical Oncology.
Importance of registry studies
Clinical trials tend to have stringent entry criteria.Even patients who are eligible to enter a clinical trial may not be willing to participate because of the more extensive study procedures, the risk of not receiving the medication they want or for a host of other reasons.These factors significantly limit the generalizability of the study results to the general population.
Registries mitigate these limitations by casting a wider net to include a wider range of patients.Thus, results of registry studies are closer to real-world situations and have greater generalizability.Two such important long-term, large-scale,longitudinal observational databases on prostate cancer are J-CaP (Japan Study Group for Prostate Cancer) from Japan and CaPSURE (Cancer of the Prostate Strategic Urologic Research Endeavor) from the United States.
J-CaP surveillance is a nationwide longitudinal observational study of patients newly starting hormone therapy for prostate cancer from January 2001 to December 2003 with more than 26,000 cases enrolled.CaPSURE on the other hand, was initiated in 1995 to document national trends in prostate cancer epidemiology, disease management, oncologic outcomes,and health-related quality of life (HRQOL) outcomes.It is a longitudinal and observational database accruing data from a total of 40 urologic practice sites over the history of the registry.It currently has around 14,000 patients in their database.
A joint initiative was established in 2007 with the objective of analyzing, reviewing, comparing and contrasting data from J-CaP and CaPSURE registries.This comparison has shown many similarities as well as differences in the treatment and treatment outcomes of prostate cancer between Japan and US10,11.
Effect of PADT and different outcomes between Japan and the United States
PADT is endorsed as an option for monotherapy for localized prostate cancer by guidelines in Asia but not in the U.S.or Europe12.PADT monotherapy is commonly used, however, in both the U.S.and Japan, especially for high risk groups in the U.S.In other Asian countries it is also commonly used11.
Data were analyzed from the CaPSURE registry representing community-based practice in the U.S., and from the J-CaP database.Risk adjustment was performed using the Japan Cancer of the Prostate Risk Assessment (J-CAPRA) score,validated specifically for men with advanced disease and those treated with PADT13(Table 2).Prostate cancer-specific mortality (PCSM), adjusting for age, J-CAPRA, year of diagnosis, and treatment type [combined androgen blockade(CAB) vs.medical or surgical castration monotherapy] were analyzed.Men treated with PADT in J-CaP were slightly older than those in CaPSURE, and had a higher risk disease.They were more likely to be treated with CAB.In the multivariable regression analysis, the hazard ratio (HR) for PCSM was 0.31 for J-CaP compared to CaPSURE.In J-CaP, CAB improved survival compared to castration monotherapy, but this effect was not observed in CaPSURE.For all-cause mortality, the HR for J-CaP was 0.27.

Table 2 Risk assessment: J-CAPRA13
Adjusting for multiple factors including disease risk and type of androgen ablation, men treated with PADT in Japan compared to the U.S.have greater than three-fold better cancer-specific survival and four-fold better overall survival.CAB improves outcomes compared to castration monotherapy in J-CaP but not in CaPSURE14.The report concluded that these findings substantiate guidelines both encouraging PADT in Asia and discouraging its use in the U.S.The reasons for these substantial differences are likely multifactorial, including both genetic and environmental factors, and elucidating them will likely yield critical insights into the biology of prostate cancer on both sides of the Pacific.
Intermittent androgen depletion (IAD)therapy and continuous androgen depletion (CAD) therapy
CAD therapy is the standard treatment for metastatic prostate cancer.CAB is the regimen most often used in Japan15.On the other hand, IAD therapy allows for testosterone levels in the blood to be recovered during the periods when it is not being administered, which contributes to improving quality of life(QOL).In addition, animal tests have suggested the possibility that IAD could delay the advance of CRPC16.In recent years ADT has come to be often used in cases of non-metastatic prostate cancer10, and in such cases there are expectations that IAD could prove to be of particular benefit.The results of a randomized clinical trial (RCT) implemented by Crook et al.17could be said to be representative of the expectations for IAD.At the same time, at the annual meeting of the American Society of Clinical Oncology (ASCO) in June 2012, the results of a long-term, large-scale RCT into IAD for metastatic prostate cancer were announced.These results provided important new information about the effect of IAD and created considerable discussion18,19.
Bene fits of IAD
According to the NCCN Clinical Practice Guidelines for prostate cancer (2013 v.4), “Intermittent ADT may reduce side effects without altering survival compared to continuous ADT, but the long-term efficacy of intermittent ADT remains unproven.”12.To date a number of RCT have compared IAD and CAD and have shown that there are no differences in overall survival time20,however, the observation period (median value) ranges from 30.8 months to approximately 6.9 years and therefore cannot be said to be sufficient.The SWOG 9345 (INT-0162) trial reported by Hussain et al.19was a large-scale, long-term test with a median observation period of 9.2 years, involving eligible cases from a total of 1,535 patients.To date this has been the study from among the published RCT that have compared IAD and CAD that has had the longest observation period and largest number of cases.As noted above, the RCT by Crook et al.17was a comparative study on non-metastatic prostate cancer and contrasted with the SWOG 9346 trial.
In this paper the results of two recent representative RCT that sought to compare IAD and CAD have been cited.It is believed that these two papers will continue to be cited in the future as important RCT in terms of both their scale and quality.At the same time, these trials examined cases of PSA relapse after radiotherapy in localized prostate cancer and also the uses of ADT for metastatic prostate cancer.
So is the use of IAD a good or a bad thing?
To sum up, IAD is linked to individualization of treatment.This may be simplistic, but at the current point this is all that can be said.The history of treatment of prostate cancer is relatively long and it has various aspects, depending on staging at the time of diagnosis and the pathological background.Although various RCTs have been implemented, it is virtually impossible to arrive at a universal conclusion with regard to the use of IAD.
This is because it is necessary to consider and deal with countless confounding factors, including patient background,therapeutic drugs, dosage, timing of the start of administration,timing of halting of administration, establishment of endpoints for trials, and balancing QOL with treatment costs, etc.
So why are such complex RCTs necessary?
The answer to this question is deeply linked to the fact that even today more than 70 years since Dr.Huggins first proposed ADT21, the basic drug therapy for prostate cancer is elimination of testes-derived testosterone.In other words, to the extent that this drug therapy is all that is available to us, we will be unable to break away from the curse of testosterone losing syndrome.The major significance of IAD is that it helps to support QOL and therefore more large-scale RCTs on IAD will be required in the future.
Based on recent results of androgen-axis research, we cannot yet allow ourselves to believe at any time soon that drug therapy for prostate cancer will be free from the fixed notion that the principle for treatment is testosterone elimination.
If it were possible to eliminate the clinical challenges presented by testosterone losing syndrome, the significance of comparing IAD and CAD would also virtually disappear.
Future concept of ADT
Prostate cancer is a classic androgen-sensitive cancer.The effectiveness of ADT at all clinical stages of prostate cancer is clear and significant.In other words, if testosterone is eliminated it is possible to easily control the disease.However, there are two significant issues that face ADT for prostate cancer.Firstly,ADT is not a radical treatment.Secondly, current ADT focuses on the elimination of testosterone.With regard to the first issue,a body of knowledge has been built up for ADT with regard to advanced cancer and metastatic cancer.Within these categories it is known that over the course of a number of years ADT becomes ineffective against many forms of prostate cancer.However, the fact that new drugs for CRPC have shown clinical effectiveness suggests that current ADT does not completely eliminate testosterone or completely inhibit the action of testosterone androgen receptors.These facts imply that in the future it will not be impossible to establish more powerful 1stline ADT.Furthermore, the possibilities for pathway control using AR receptors suggest that the adverse effects of testosterone elimination, namely testosterone losing syndrome, could be avoided in the future.It is believed that the role of ADT in prostate cancer treatment will become increasingly important.
Conflict of interest statement
No potential conflicts of interest are disclosed.
1.Akaza H, Naito S, Cheng C, Kaisary A, Soebadi DM, Umbas R,et al.Asian trends in prostate cancer hormone therapy.Gan To Kagaku Ryoho 2002;29:1951-1961.
2.Akaza H, Chang SJ, Chen KK, Esuvaranathan K, Fujioka T, Hirao Y, et al.The 2nd conference on Asian trends in prostate cancer hormone therapy.Gan To Kagaku Ryoho 2003;30:1533-1542.
3.Akaza H, Naito S, Chang SJ, Chen KK, Cheng C, Choi HY, et al.The 3rd Conference on Asian Trends in Prostate Cancer HormoneTherapy.Gan To Kagaku Ryoho 2004;31:1285-1295.
4.Akaza H, Moore MA, Chang SJ, Cheng C, Choi HY, Esuvaranathan K, et al.The 5th Conference on Asian Trends in Prostate Cancer Hormone Therapy.Asian Pac J Cancer Prev 2007;8:3-12.
5.Cheng C, Akaza H, Chen KK, Moore MA, Naito S, Song JM, et al.Prostate cancer control--aims of the UICC Asia Regional Office Consortium.Asian Pac J Cancer Prev 2006;7:350-368.
6.Namiki M, Akaza H, Lee SE, Song JM, Umbas R, Zhou L, et al.Prostate Cancer Working Group report.Jpn J Clin Oncol 2010;40 Suppl 1:i70-75.
7.Available online: http://www.linkedin.com/pub/asian-pacificprostate-society/56/94a/352
8.Available online: http://demo.nccn.org/professionals/physician_gls/PDF/kidney-asia.pdf
9.Available online: http://demo.nccn.org/professionals/physician_gls/PDF/prostate-asia.pdf
10.Akaza H, Carroll P, Cooperberg MR, Hinotsu S.Fifth Joint Meeting of J-CaP and CaPSURE: advancing the global understanding of prostate cancer and its management.Jpn J Clin Oncol 2012;42:226-236.
11.Akaza H, Hinotsu S, Cooperberg MR, Chung BH, Youl Lee J,Umbas R, et al.Sixth joint meeting of J-CaP and CaPSURE--a multinational perspective on prostate cancer management and patient outcomes.Jpn J Clin Oncol 2013;43:756-766.
12.Available online: http://demo.nccn.org/professionals/physician_gls/pdf/prostate.pdf
13.Cooperberg MR, Hinotsu S, Namiki M, Ito K, Broering J, Carroll PR, et al.Risk assessment among prostate cancer patients receiving primary androgen deprivation therapy.J Clin Oncol 2009;27:4306-4313.
14.Matthew R.Cooperberg M, Hinotsu S, Namiki M, Carroll P,Akaza H, et al.Trans-pacific variation in outcomes for men treated with primary androgen deprivation therapy for localized prostate cancer.AUA 2013 Annual Meeting (SanDiego) abstract #724.
15.Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S, et al.Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind,randomized study for survival.Cancer 2009;115:3437-3445.
16.Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD.Effects of intermittent androgen suppression on androgen-dependent tumors.Apoptosis and serum prostatespecific antigen.Cancer 1993;71:2782-2790.
17.Crook JM, O’Callaghan CJ, Duncan G, Dearnaley DP, Higano CS,Horwitz EM, et al.Intermittent androgen suppression for rising PSA level after radiotherapy.N Engl J Med 2012;367:895-903.
18.ASCO Daily News 2012 Annual Meeting Wrap Up.Continuous androgen-deprivation therapy remains standard care for metastatic prostate cancer.SWOG 9346 (INT-1062), (ASCO 2012 abstract #4).
19.Hussain M, Tangen CM, Berry DL, Higano CS, Crawford ED, Liu G, et al.Intermittent versus continuous androgen deprivation in prostate cancer.N Engl J Med 2013;368:1314-1325.
20.Mitin T, Efstathiou JA, Shipley WU.Urological cancer.The bene fits of intermittent androgen-deprivation therapy.Nat Rev Clin Oncol 2012;9:672-673.
21.Huggins C, Hodges CV.Studies on prostatic cancer: I.The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate.Cancer Res 1941;1:293-297.
 Cancer Biology & Medicine2013年4期
Cancer Biology & Medicine2013年4期
- Cancer Biology & Medicine的其它文章
- Rare myeloid sarcoma/acute myeloid leukemia with adrenal mass after allogeneic mobilization peripheral blood stem cell transplantation
- Analysis of 30 patients with persistent or recurrent squamous cell carcinoma of the cervix within one year after concurrent chemoradiotherapy
- Effects of HLEC on the secreted proteins of epithelial ovarian cancer cells prone to metastasize to lymph nodes
- Why bortezomib cannot go with ‘green’?
- Therapeutic resistance in cancer: microRNA regulation of EGFR signaling networks
- Translational genomics in cancer research: converting pro files into personalized cancer medicine
