基于三核锰バ结构单元的二维配位聚合物的合成、结构和磁性研究
郭利芳 王兆喜*,,2 李明星*,
(1上海大学理学院化学系,上海 200444)
(2温州大学化学与材料工程学院,温州 325027)
0 Introduction
Coordination polymers and metal-organic frameworks based on polycarboxylate ligands have attracted great interest owing to their intriguing structural motifs[1-4]and potential applications such as separation[5],catalysis[6],magnetism[7],and gasstorage[8-10].In particular,benzenedicarboxylic acids are widely used in the preparation of coordination polymers owing to their rich coordination modes.The derivatives of benzenedicarboxylic acid with substituent groups such as methyl[11],chlorine[12],and bromine[13]are signicant to assemble coordination polymers with transition-metals.Moreover,organo-fluorine compounds have been widely used in pharmaceutical,agrochemical and catalytic materials,due to the peculiar characteristics of fluorine element including high electronegativity, low polarizability and small covalent radius[14-21].Tetrauoroterephthalic acid,as a fluorine-containing versatile ligand and acceptor/donor of hydrogen bond,is a good candidate for the construction of coordination polymers with interesting structures and peculiar properties.Theoretical investigation predicts that coordination polymers using 2,3,5,6-tetrafluoroterephthalate as linker have superior H2adsorbing properties[22-24].To date,several coordination polymers employing tetrauoroterephthalate (tfbdc2-)have been reported[25-37].Previous studies were mainly focused on single metal center connected by tetrauoroterephthalate.In this work,we report the preparation,structure and magnetism of a novel 2D coordination polymer based on trinuclear[Mn3O]cluster bridged by tetrauoroterephthalate and sodiumcation.
1 Experimental
1.1 Materials and measurements
All chemicals were of reagent grade and used as received without further purification.Elemental analyses for carbon,hydrogen,and nitrogen were carried out with a Vario EL-Ⅲ elemental analyzer.Infrared spectrum was recorded with a Nicolet A370 FTIR spectrometer using KBr pellets in the 400~4 000 cm-1region.TGA experiment was performed with a Shimadzu DT-20B thermo gravimetric analyzer from 20 to 800℃ at a heating rate of 10℃·min-1in nitrogen.Variable-temperature magnetic susceptibility measurement was taken at an applied field of 2 kOe on a Quantum Design MPMS-XL7 SQUID magnetometer working in 300~1.8 K temperature range.Diamagnetic correctionswereapplied by using Pascalsconstants.
1.2 Synthesis of{[NaMnⅢ3O(sao)3(tfbdc)(H 2O)4]·0.5H 2O·2CH 3CH 2OH}n(1)
A mixture of MnCl2·4H2O (20.0 mg,0.1 mmol),2,3,5,6-tetrafluoroterephthalic acid (11.9 mg,0.05 mmol)and salicylaldoxime (13.7 mg,0.1 mmol)were dissolved in amixed solvent of water(3 mL)and ethanol(6 mL)with stirring.Additional NaOH solution(1 mol·L-1)was added to adjust the value of pH to 8,and the mixture turned dark green.After stirring for 10 min,the mixture was filtered and the filtrate was left undisturbed three weeks to give dark-green block crystals of 1 in 30% yield based on Mn. Anal. Calcd. for C66H72F8Mn6N6Na2O35(%):C,38.91;H,3.56;N,4.12.Found(%):C,38.56;H,3.52;N,3.96.IR data(KBr,cm-1):3 500(s),1 622(s),1 597(s),1 543(m),1 472(m),1 440(m),1 374(s),1 280(s),1 029(m),987(m),919(s),679(s),648(m).
1.3 X-ray crystallography
The well-shaped single crystal of 1 was selected for X-ray diffraction study.Data collections were performed with graphite-monochromatized Mo Kα radiation(λ=0.071 073 nm)on a Bruker Smart Apex-II CCD diffractometer,using the φ-ω scan technique.Data reduction was made with the Bruker SAINTpackage.Absorption correction wasperformed usingthe SADABSprogram.The structures were solved by direct methods and refined by full-matrix least-squares on F2with SHELXTL-2000 program package[38].All nonhydrogen atoms were refined anisotropically,and hydrogen atoms were located and included at their calculated position.Crystallographic data and detailson refinements are summarized in Table 1.Selected bond distances and angles are listed in Table 2.Hydrogen bondsaregiven in Table 3.
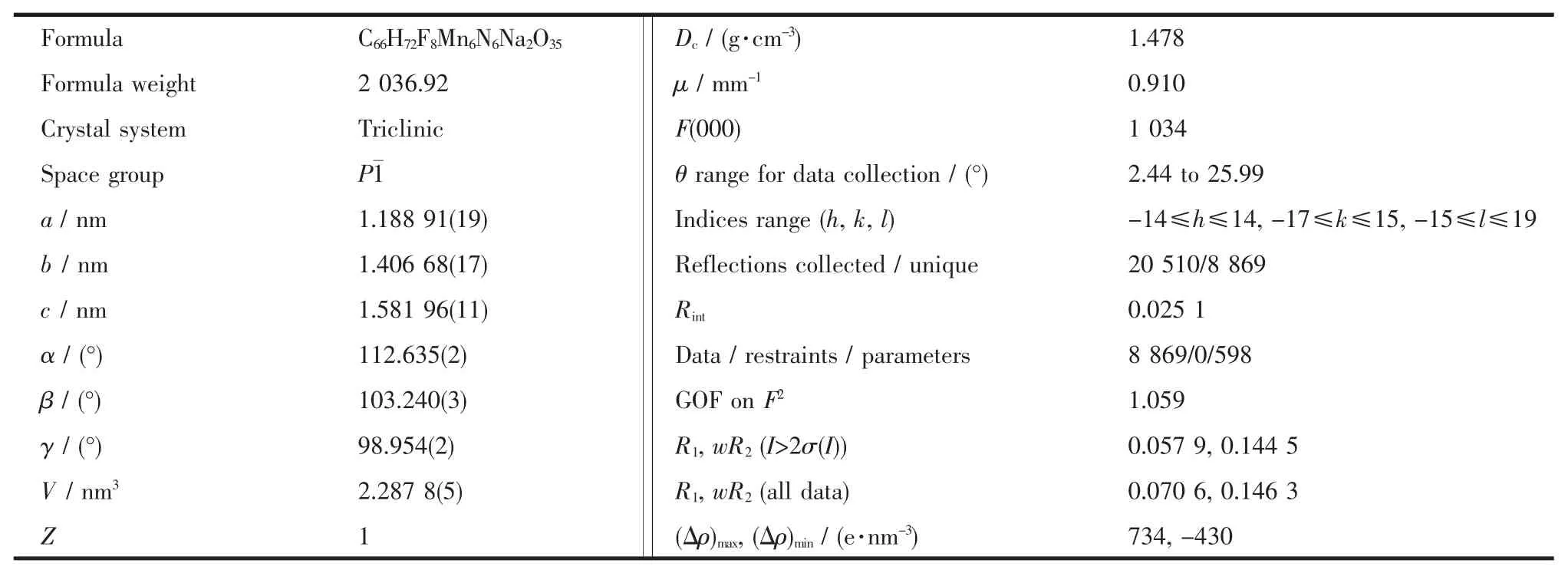
Table 1 Crystal data and structure refinements for complex 1
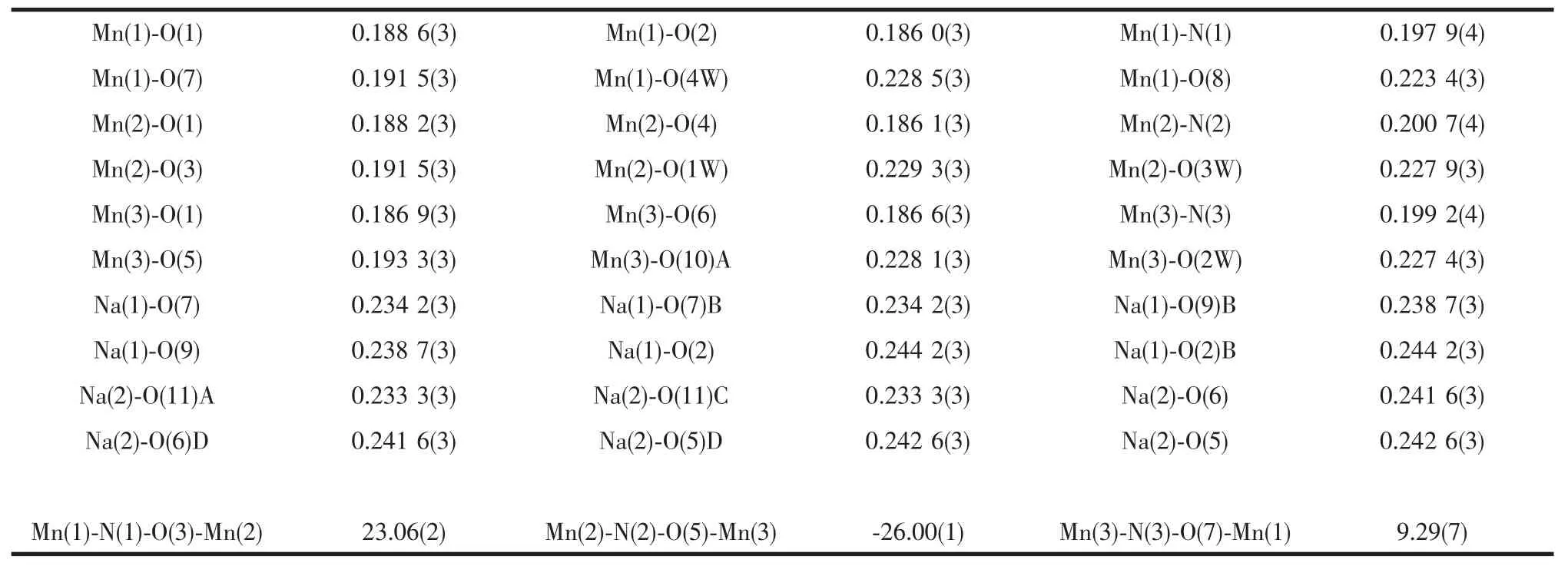
Table 2 Selected bond lengths(nm)and angles(°)for complex 1
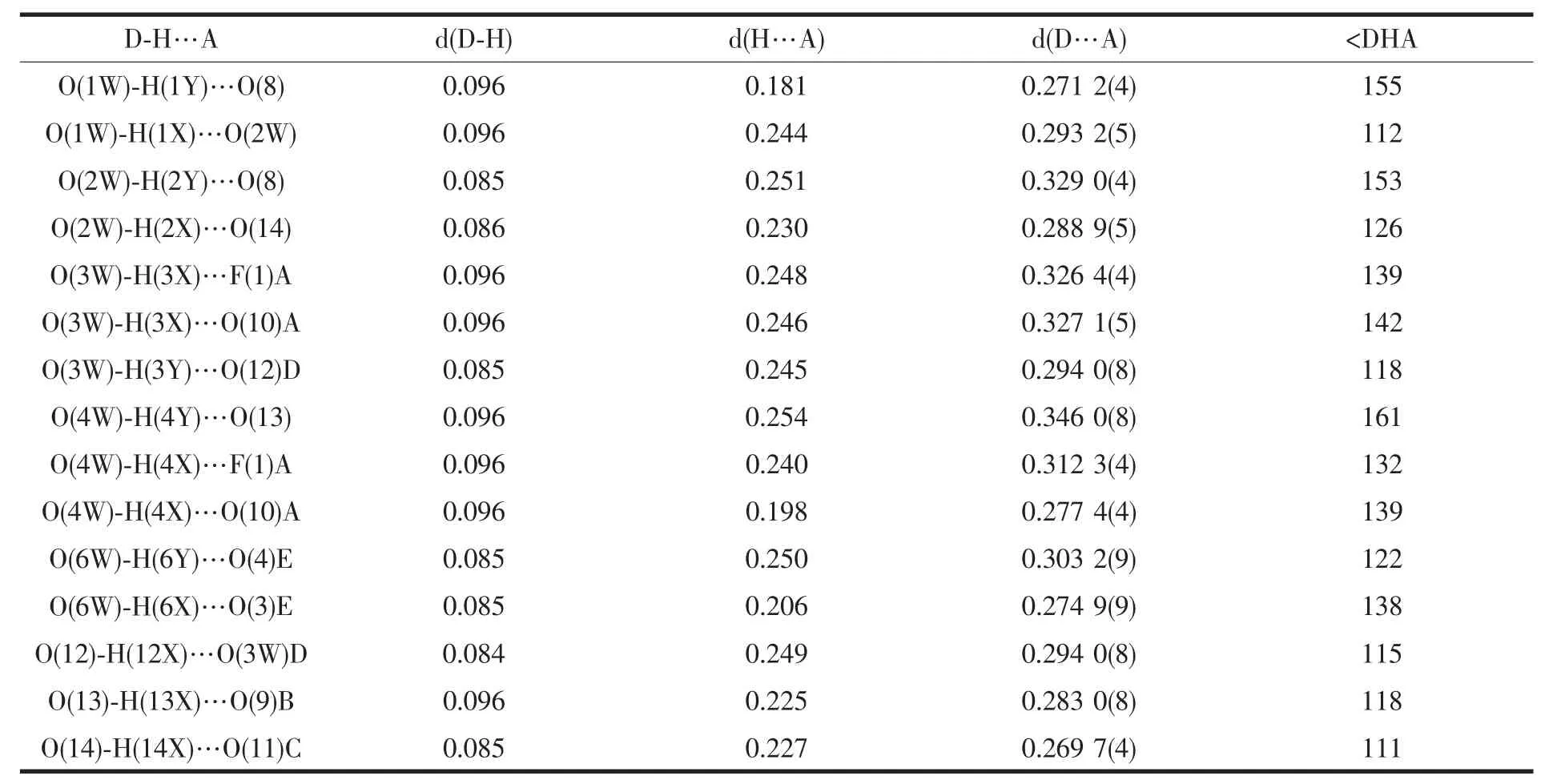
Table 3 Hydrogen bonding parameters for complex 1
CCDC:934761.
2 Resultsand discussion
2.1 Description of thecrystal structure
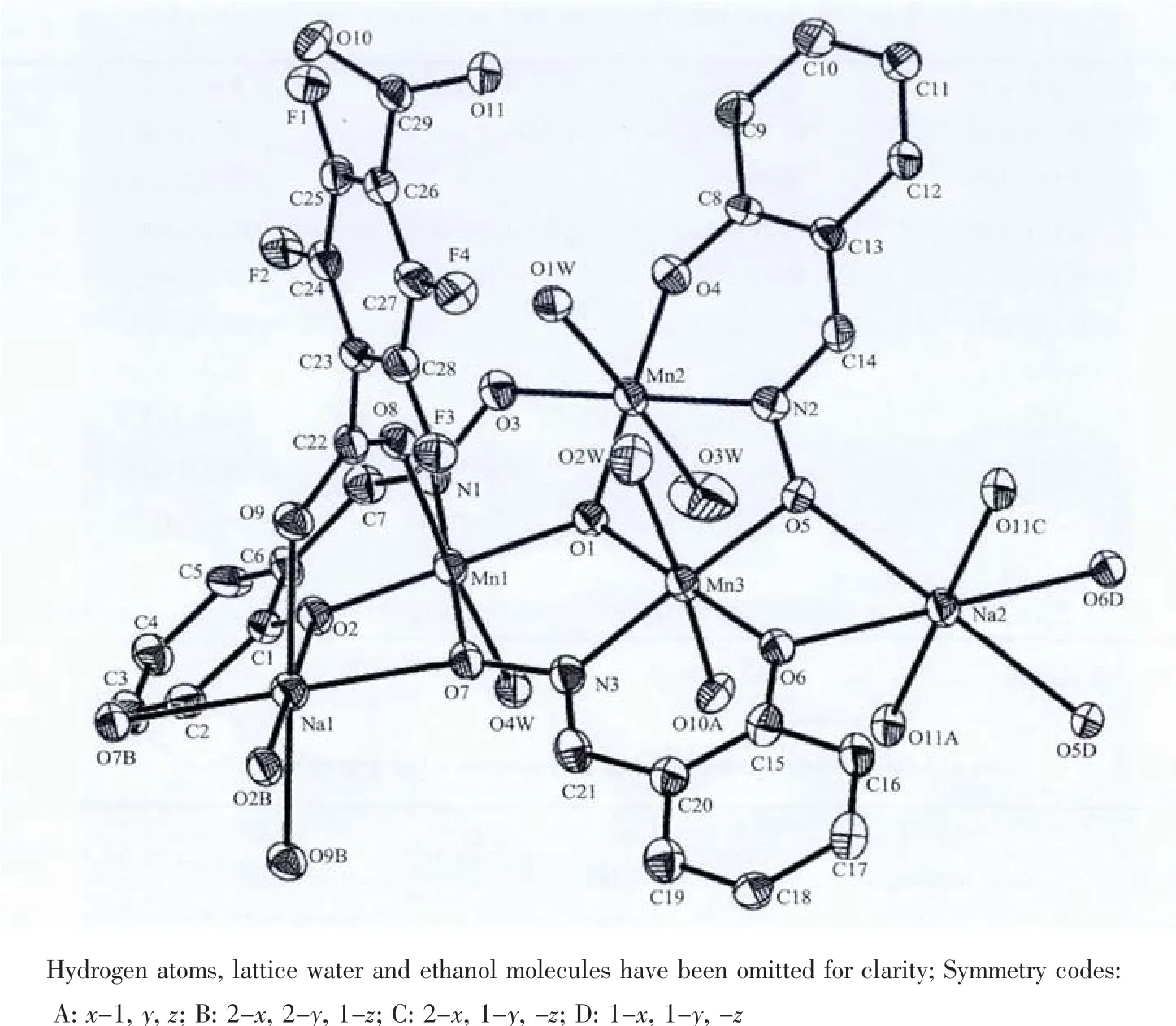
Fig.1 Asymmetric unit and coordination environment of Mnバin 1 with ellipsoids drawn at 30%probability level
Single-crystal X-ray structural analysis reveals that complex 1 crystallizes in triclinic space group P1.The complex 1 comprises a two-dimensional polymeric framework based on triangular-shaped [Mn3O]units linked by tfbdc2-and Na+cations to each other.The tfbdc2-ligand acts as aμ2-bridge and connects two MnIIIions and two Na+ions through carboxyl oxygen donors.As shown in Fig.1,the asymmetric unit contains three MnⅢions,one Na+,one tfbdc2-and three sao2-ligands together with four coordinated water molecules,half of a lattice water molecule and two ethanol molecules.The Mn(1),Mn(2)and Mn(3)centers adopt similar distorted octahedral geometry,six-coordinated by five oxygen atoms and one nitrogen atom.The four coordinate sits on equatorial plane in each MnIIIoctahedron are occupied by two oxygen atoms and one nitrogen atom from two sao2-ligands as well as the center oxide O(1).However,the Jahn-Teller axises of three Mnバoctahedra are obviously different.The axial positions of Mn(2)are occupied by two coordinate water molecules,while Mn(1)is occupied by coordinated water O(4W)and carboxyl O(8).Similar to Mn(1),the axial positions of Mn(3)are occupied by coordinated water O(2W)and carboxyl O(10A).The equatorial Mn-Oand Mn-Nbond lengthsaround three Mnバatomsare about 0.190 and 0.200 nmrespectively.While the axial Mn-Odistancesaresignicantly longer(from0.223 4(3)to 0.229 3(3)nm,see Table 2).Thisphenomenon is also found in reported Mnバcomplexes[39].Interestingly,the Na(1)and Na(2)cations also adopt a six-coordinated octahedral geometry,bound by two phenoxo oxygen and two oximato oxygen donors from four sao2-ligands as well as two carboxyl oxygen atoms from two tfbdc2-,which is similar to one complex reported by us[40].In the complex 1,the asymmetric unit contains two half Na+cation,Na(1)and Na(2),which is different from the reported complex.The Na-Obond distances range from 0.233 3(3)to 0.244 2(3)nm,which are normal as expected[41].In addition,the Mn-N-O-Mn torsion angles are 23.06(2)°,-26.00(1)°and 9.29(7)°,which are important parameters to influence magnetic couple between Mnバcenters.Both terminal carboxyl groups of 2,3,5,6-tetrafluoroterephthalate adopt monodentate coordination mode.The tetrauorinated phenyl ring of tfbdc2-is inclined to the carboxylate group with the dihedral angles of 55.947°and 56.809°.This was attributed to an electrostatic repulsion between the fluorine atoms and the carboxyl oxygen atoms as well as a decrease in aromatic character of the carboxyl group due to the electron-withdrawing nature of the fluorine atoms[42-43].
In complex 1,sodium cation as bridge links adjacent[Mn3O]units to forma 1D zigzag chain(Fig.2).Furthermore,the neighboring 1D array is connected by tfbdc2-ligands to form a 2D coordination polymer(Fig.3a).In the 2D polymeric network,the[Mn3O]units as nodes construct a four-connected (4,4)topologic network (Fig.3b).In the crystal structure,several intramolecular and intermolecular hydrogen bonds are observed (Table 3),originating from the interactions between fluorine atoms,carboxyl oxygen,coordinated water and lattice water.All the H-bonding interactions assemble the 2D polymeric network of 1 into a 3D supramolecular architecture.
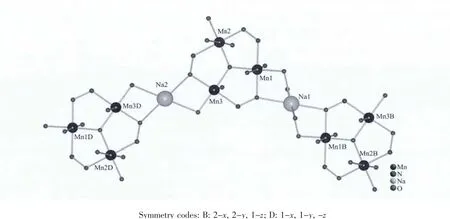
Fig.2 Sodium-bridging 1D zigzag chain formed by[Mn3O]units
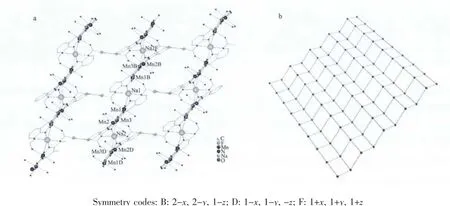
Fig.3 Structure of 2D polymeric network(a),and the(4,4)topologic network in which[Mn3O]units as nodes(b)
2.2 IR spectrum
The IR spectrum of complex 1 exhibits the characterization absorptions of salicylaldoxime,H2tfbdc and water.The broad absorption band near 3 500 cm-1corresponds toν(O-H)of the coordinate and lattice water molecules.The peaks at 1 597,1 543,1 471,1 440 cm-1indicate existence of salicylaldoxime.No absorption peak around 1 690~1 730 cm-1indicates that the 2,3,5,6-tetrafluoroterephthalic acid is deprotonated.The strong peaks at 1622 and 1374 cm-1are assigned the asymmetric stretching vibrationνas(COO-)and the symmetric stretching vibration νs(COO-)of tfbdc2-.The 248 cm-1difference between νas(COO-)and νs(COO-)indicates that the tfbdc2-ligand adopts monodentate coordination[44],as proved by X-ray crystal structural analysis.In addition,the strong peak at 917 cm-1is the δ(COO-)bent vibration of tfbdc2-[45].
2.3 Thermogravimetric analysis
Thermal stability of complex 1 has been investigated by TG technique and the thermogravimetric curve is shown in Fig.4.TGanalysis shows that the lattice water and ethanol molecules are eliminated in thetemperature rangeof 60~70 ℃ (Calcd.9.94%;Found:10.06%).The following weight-loss occured from 70 to 190℃is attributed to the loss of four coordinated water molecules (Calcd.7.06%;Found:7.07%).Then 2,3,5,6-tetrafluoroterephthalate ligand is eliminated when heating from 190 to 270℃(Calcd.23.18%;Found:23.23%).The decomposition of salicylaldoximes begins at 270℃,without stopping until 800℃.
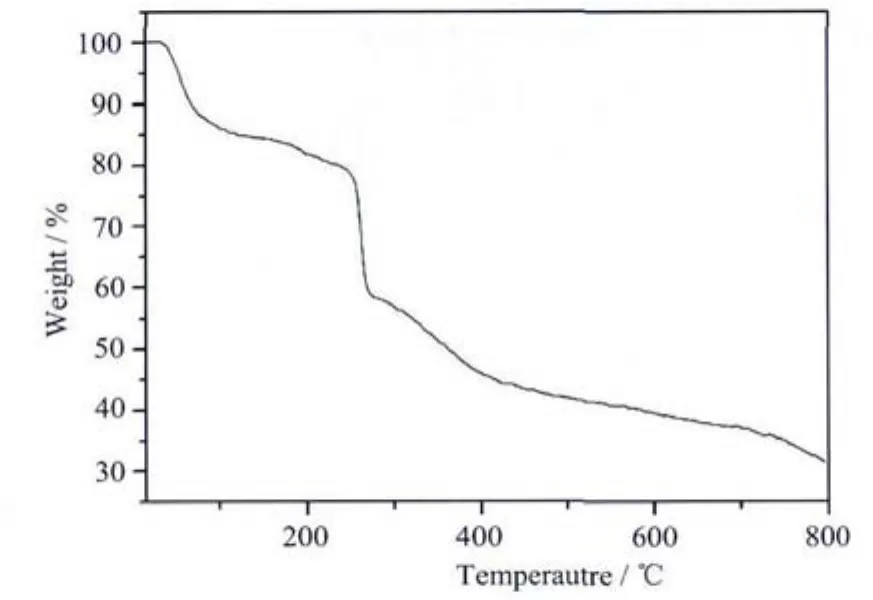
Fig.4 Thermogravimetric curve of complex 1
2.4 Magnetic Properties
Magnetic susceptibility measurements of crystalline sample of 1 were carried out using a Quantum Design MPMS-XL7 SQUID magnetometer in an applied magnetic field of 2 kOe in the temperature range of 1.8~300 K.The results are shown in the form ofχMT versus T(Fig.5).The χMT value of 1 is 8.76 emu·K·mol-1at room temperature,lower than the spin-only value of 9.0 emu·K·mol-1expected for three high-spin non-interacting MnⅢions(S=2 and g=2).When the temperature is lowered,χMT smoothly decreases above 130 K.Below 130 K,χMT shows a sharp drop to a value of 1.79 emu·K·mol-1at 1.8 K,which is typical for antiferromagnetic interactions[46-47].In order to further investigate the magnetic interaction between the MnⅢions in the triangular-shaped [Mn3O]cluster,the magnetic data are simulated by the isotropic spin Hamiltonian H=-2J1(SMn1SMn2+SMn1SMn3)-2J2SMn2SMn3,allowed us to satisfactorily simulate the data with the parameters J1=-1.06 cm-1,J2=-2.81 cm-1,g=2.05.The agreement factor R,defined here as R=∑[(χMT)calc-( χMT)obs]2/∑( χMT)obs2,is equal to 7.31×10-3.Normally,interaction through the oximato bridges is antiferromagnetic couple in such complexes due to the low Mn-N-O-Mn torsion angles,which is in good agreement with the magneto-structural correlations found in oxide-centered triangle[MnⅢ3]cluster[48].
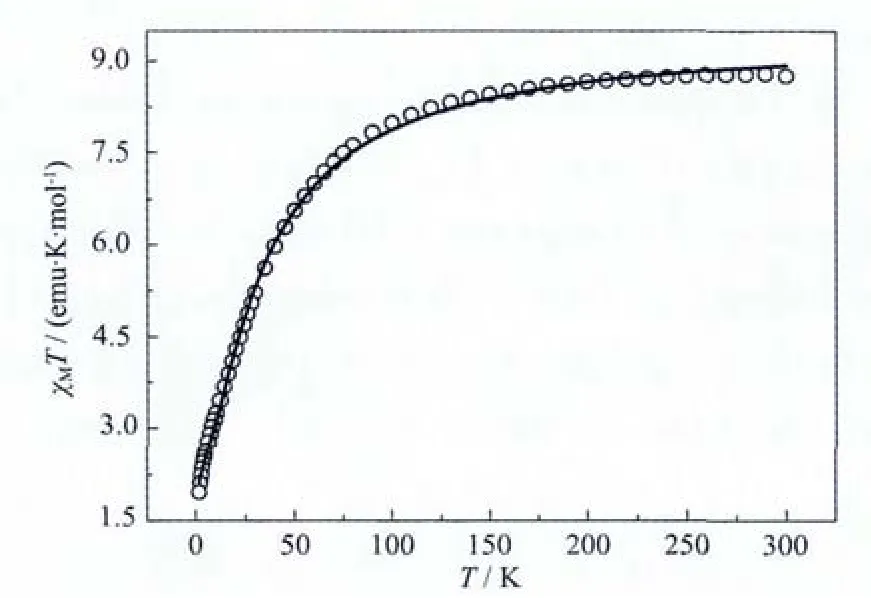
Fig.5 Temperature dependence of magnetic susceptibili ties of 1 in an applied eld of 2000 Oe
[1]Cheetham A K,Férey G,Loiseau T.Angew.Chem.Int.Ed.,1999,38:3268-3292
[2]Rao CNR,Natarajan S,Vaidhyanathan R.Angew.Chem.Int.Ed.,2004,43:1466-1496
[3]Kitagawa S,Kitaura R,Noro S.Angew.Chem.Int.Ed.,2004,43:2334-2375
[4]Pan L,Olson D H,Ciemnolonski L R,et al.Angew.Chem.Int.Ed.,2006,45:616-619
[5]Mueller U,Schubert M,Teich F,et al.J.Mater.Chem.,2006,16:626-636
[6]Rosseinsky M J.Microporous Mesoporous Mater.,2004,73:15-30
[7]Maspoch D,Ruiz-Molina D,Veciana J.J.Mater.Chem.,2004,14:2713-2723
[8]Collins D J,Zhou H C.J.Mater.Chem.,2007,17:3154-3160
[9]Morris R E,Wheatley P S.Angew.Chem.Int.Ed.,2008,47:4966-4981
[10]Ma S,Zhou H C.Chem.Commun.,2010,46:44-53
[11]Braun M E,Steffek C D,Kim J,et al.Chem.Commun.,2001:2532-2533
[12]Chen S C,Zhang Z H,Huang K L,et al.Cryst.Growth Des.,2008,8:3437-3445
[13]Li C P,Tian Y L,Guo Y M.Inorg.Chem.Commun.,2008,11:1405-1408
[14]Vela J,Smith J M,Yu Y,et al.J.Am.Chem.Soc.,2005,127:7857-7870
[15]Ohashi M,Kambara T,Hatanaka T,et al.J.Am.Chem.Soc.,2011,133:3256-3259
[16]Chan P W Y,Yakunin A F,Edwards E A,et al.J.Am.Chem.Soc.,2011,133:7461-7468
[17]Ge C,Zhang X,Yin J,et al.Chin.J.Chem.,2010,28:2083-2088
[18]Jana A,Roesky H W,Schulzke C,et al.Organometallics,2010,29:4837-4841
[19]Beltrán T F,Feliz M,Llusar R,et al.Organometallics,2011,30:290-297
[20]Lemal D M.J.Org.Chem.,2004,69:1-11
[21]Sandford G.Tetrahedron,2003,59:437-454
[22]Zhang L,Wang Q,Liu Y C.J.Phys.Chem.B,2007,111:4291-4295
[23]Hübner O,Glöss A,Fichtner M,et al.J.Phys.Chem.A,2004,108:3019-3023
[24]Yang C,Wang X,Omary M A.J.Am.Chem.Soc.,2007,129:15454-15455
[25]Cotton FA,Lin C,Murillo CA.J.Chem.Soc.,Dalton Trans.,1998:3151-3154
[26]Chisholm M H,Wilson PJ,Woodward PM.Chem.Commun.,2002:566-567
[27]Rau S,Böttcher L,Schebesta S,et al.Eur.J.Inorg.Chem.,2002:2800-2809
[28]Kitaura R,Iwahori F,Matsuda R,et al.Inorg.Chem.,2004,43:6522-6524
[29]Ito M,Onaka S.Inorg.Chim.Acta,2004,357:1039-1046
[30]Chun H,Dybtsev D N,Kim H,et al.Chem.Eur.J.,2005,11:3521-3529
[31]Chen B,Yang Y,Zapata F,et al.Inorg.Chem.,2006,45:8882-8886
[32]Yoon JH,Choi SB,Oh Y J,et al.Catal.Today,2007,120:324-329
[33]ZHU En-Jing(朱 恩 静),LIU Qi(刘 琦),CHEN Qun(陈 群),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(9):1428-1433
[34]YU Li-Li(于丽丽),LIU Qi(刘琦),XI Hai-Tao(席海涛),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(4):621-626
[35]Hulvey Z,Falcao E H L,Eckert J,et al.J.Mater.Chem.,2009,19:4307-4309
[36]Hulvey Z,Ayala E,Furman J D,et al.Cryst.Growth Des.,2009,9:4759-4765
[37]Hulvey Z,Ayala E,Cheetham A K.Z.Anorg.Allg.Chem.,2009,635:1753-1757
[38]Bruker 2000,SMART(Version 5.0),SAINT-plus(Version 6),SHELXTL(Version 6.1)
[39]Miyasaka H,Takayama K,Saitoh A,et al.Chem.Eur.J.,2010,16:3656-3662
[40]Geng J P,Wang Z X,He X,et al.Inorg.Chem.Commun.,2011,14:997-1000
[41]Biswas B,Khanra S,Weyhermüller T,et al.Chem.Commun.,2007:1059-1061
[42]Hulvey Z,Furman J D,Turner S A,et al.Cryst.Growth Des.,2010,10:2041-2043
[43]Wang Z,Kravtsov V C,Walsh R B,et al.Cryst.Growth Des.,2007,7:1154-1162
[44]Deacon G B,Phillips R J.Coord.Chem.Rev.,1980,33:227-250
[45]Wu Y,Yu L,Cheng M,et al.Chin.J.Chem.2012,30:1045-1051
[46]Wang X Y,Sevov SC.Chem.Mater.,2007,19:3763-3766
[47]Coronado E,Galán-Mascarós J R,Marti-Gastaldo C,et al.Inorg.Chem.,2008,47:6829-6839
[48]Milios C J,Inglis R,Vinslava A,et al.J.Am.Chem.Soc.,2007,129:12505-12511
———理学院