β-二亚胺配体支持的铝氧硼六元环化合物的合成、表征及其热稳定性研究
郝朋飞 杨 智 马小莉 李加荣
(北京理工大学化工与环境学院,北京 100081)
0 Introduction
In 1960s,chemists had drawed much attention to aluminum complexes,which can be used as the active catalyst in the polymerization of epoxides[1-3],aldehydes[4-5],and olefins.It is noteworthy to mention that the typical reactions of the polymerization were catalyzed by the aluminum complexes[2,15].In 1980,Sinn et al found that the methylalumoxane(MAO,[MeAlO]n)being a highly active cocatalyst for group 4 metallocenes,catalyzing ethylene and propylene polymerization[6-7].From then on,aluminum complexes with the desirable chemical properties and structures which were shown to have various applications,for example:they are used as ion exchange materials,catalysts,catalyst supports,molecular sieves,flame retardants and sensors[8-11].In recently years,aluminum complexes exhibit excellent initiator properties for the ring opening polymerization of lactones[12-16].It is noteworthy to mention that the boroxines are very important for fundamental academic,for example:structural investigations,electrochemistry,intermediate products,etc.What's more it is also important for industrial applications (flame retardants for light metals,lithium ion battery materials,etc.)[17].So the aluminum substituted boroxines containing the Al-O-B moiety might have unusual properties compared to those of boroxines.However,compounds containing the Al-O-Bmoiety are very rare.In 2006[18]and in 2011[19],we reported a series of novel structures bearing the Al-O-B unit by the reaction of organic aluminum precursors with organic compounds containing B-OH groups.
Herein, according to our recent research results[20-21],wereport on thealuminumboroxinecomplex by thereaction of LAlH2{L=(Z)-4-[(2,6-diisopropylphenyl)amino]pent-3-en-2-ylidene-2,6-diisopropylaniline}with2-trifluoromethylphenylboronic acid.
1 Experimental
1.1 General Procedures
All manipulations were carried out under a purified nitrogen atmosphere using Schlenk techniques or inside a Mbraun MB 150-GI glovebox.All solvents were distilled from Na/benzophenone ketyl prior to use.Commercially available chemicals were purchased from Aldrich,Fluka and used as received.LH[22]and LAlH2[23]were prepared as described in literature.Elemental analyse was carried out on an Elemental Vario EL analyzer at the Analytical Instrumentation Center of the Peking University.1H NMR spectrum were recorded on Bruker AM 400 spectrometer.Infrared spectra were recorded on a Perkin Elmer spectrophotometer.Melting points were measured in sealed glass tubes.
1.2 Synthesis
1.2.1 Synthesis of LAl[OB(2-CF3C6H4)]2(μ-O)
A solution of LAlH2(0.446 g,1.0 mmol)in toluene(10 mL)was added drop by drop to a solution of 2-trifluoromethylphenylboronic acid(0.380 g,2 mmol)in toluene(10 mL)at 0 ℃ (Scheme 1).After the addition wascomplete,the reaction mixture wasallowed to warm to room temperature and stirring was continued overnight.The solvent was removed in vacuo.The solid was extracted with n-hexane (30 mL),and the extract was stored at room temperature for 2 d to afford target compound as colorless crystals.An additional crop of target compound was obtained from the mother liquor.Total yield:0.718 g(87.3%),mp 103~105 ℃.1H NMR(399.13 MHz,CDCl3,25 ℃,TMS):δ 7.81~7.12(m,14 H,Ar-H),5.48(s,1 H,γ-H),3.44(sept,3JH-H=6.8 Hz,4 H,CHMe2),1.60(s,6 H,Me),1.33(d,3JH-H=6.8 Hz,12 H,CHMe2),1.24 (d,3JH-H=6.8 Hz,12 H,CHMe2).IR(KBr): ν:3 062.54,2 966.11,2 873.54,1 554.41,1 521.63,1 463.77,1 384.70,1 313.34,1 284.41,1 137.84, 1 033.70, 919.92, 775.28 cm-1.C43H49AlB2F6N2O3(804.44)Calcd.(%):C,64.20;H,6.14;N,3.48.Found(%):C,64.05;H,6.19;N,3.51.

Scheme 1 Preparation of title compound
1.3 Single Crystal X-ray Structure Determination and Refinement
Single crystal of compound was mounted with glue on glass fiber and crystal data were collected on the Rigaku AFC10 Saturn724+(2×2 bin mode)diffractometer equipped with graphite-monochromated Mo Kα radiation (λ =0.071 0747 nm).Empirical absorption correction was applied using the SADABS program.[24]Structure was solved by direct methods[25]and refined by full-matrix least squares on F2using the SHELXL-97 program.[26]All non-hydrogen atoms were refined anisotropically,and the hydrogen atoms were generated geometrically and treated by a mixture of independent and constrained refinement.The crystal data and refinement details for compound is listed in Table 1,and the selected bond lengths(nm)and angles(°)are given in Table 2.
CCDC:918118.
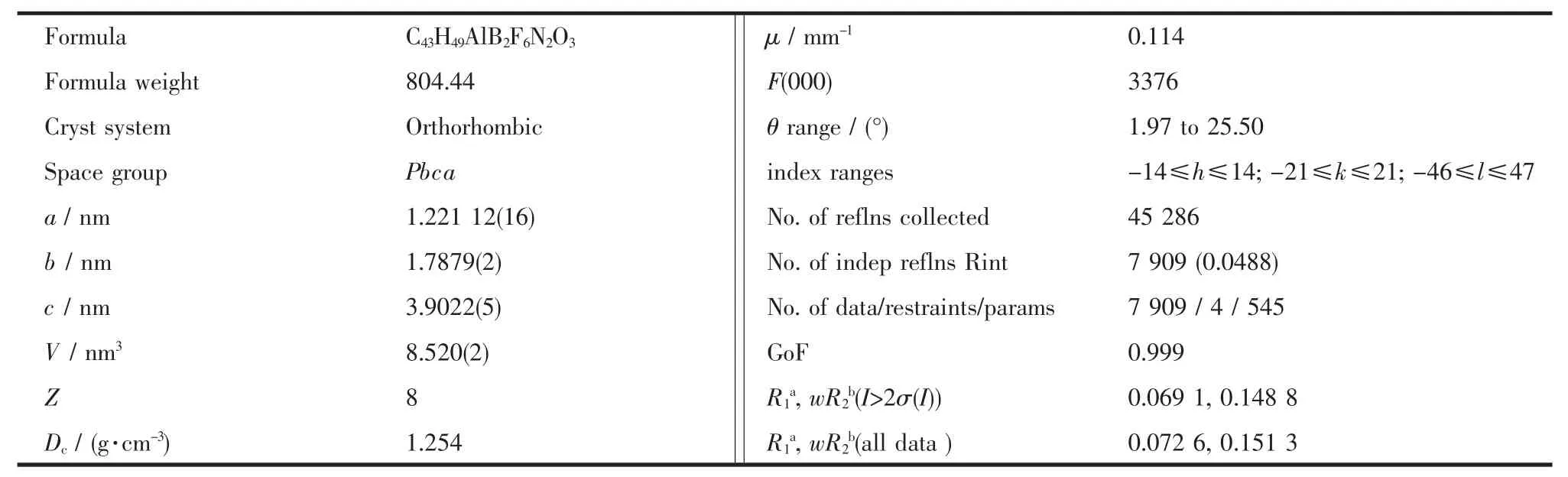
Table 1 Crystallographic data for title compound
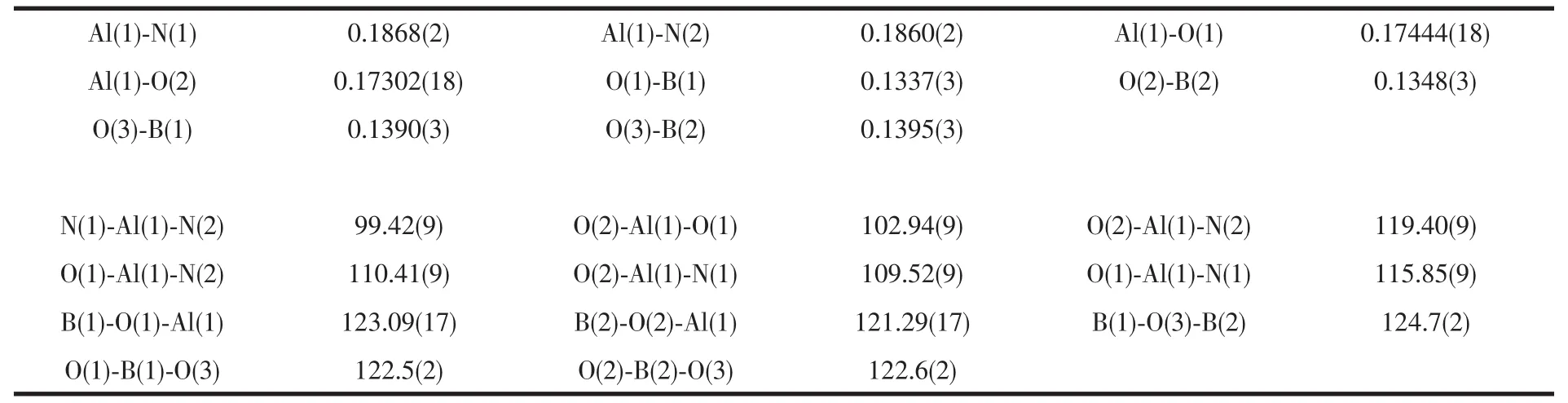
Table 2 Selected bond distances(nm)and angles(°)for title compound
2 Results and discussion
The reaction of LAlH2with 2-trifluoromethylphenylboronic acid in a molar ratio of 1∶2 resulted in the product LAl[OB(2-CF3C6H4)]2(μ-O).During the course of the reaction hydrogen gas evolution was observed,which proceeds under the elimination of 2 equiv of hydrogen.Compound was isolated after growing colorless crystals from the concentrated n-hexane solution.Compound is soluble in common solvents such as toluene,benzene,trichloromethane,and tetrahydrofuran.
2.1 Spectrum characterization of LAl[OB(2-CF3C6H 4)]2(μ-O)
Compound was characterized by1H NMR investigation in CDCl3solution,as well as elemental analysis.The1H NMR spectrum of exhibits one set of resonances for the aryl group both on boron and the ligand,indicating a symmetric molecule.Compound shows the γ-H resonances at δ5.48 ppm in an approximate of ratio of 1∶4 to that of the CHMe2at δ 3.44 ppm.In addition,the IR spectrum of compound shows a middle strong band atν2 966.11 cm-1,which is attributed to the stretching vibration of saturated hydrocarbon,while the band at 3 062.54 cm-1was assigned to the asymmetric stretching vibration of the unsaturated hydrocarbon.
2.2 Crystal structure of the crystalline LAl[OB(2-CF3C6H 4)]2(μ-O)

Fig.1 Molecular structure of title compound.Thermal ellipsoids are drawn at 30%level
X-ray quality single crystal of the compound was obtained in n-hexane at 0℃.Compound crystallizes in orthorhombic space group Pbca.The molecular structure is shown in Fig.1.The aluminum atom is four coordinated and surrounded by two bridging oxygen atoms and two nitrogen atoms,respectively.The Al-O bond lengths of the compound (av 0.1737 nm),which are comparable with the similar structure reported by the literature[20],are little longer than those of normal Al-OH bond distances(av 0.170 5 nm)in LAl(OH)2[27].The Al-N bond distances(av 0.148 64 nm)and the N-Al-N angle(99.42(9)°)are basically in line with literature[18-19].The O(2)-Al(1)-N(1)angle(109.52(9)°)is quite close to O(1)-Al(1)-N(2)(110.41(9)°),which is shorter than the angles of O(1)-Al(1)-N(1)(115.85(9)°)and O(2)-Al(1)-N(2)(119.40(9)°).In the meanwhile,the O(2)-Al(1)-O(1)angle of compound(102.94(9)°)is more or less when compared with the O(2)-Al(1)-O(3)angle of the similar structure reported by the literature (104.7(3)°)[18].The sum of the inner angles of the AlB2O3six-membered ring is 717.12°,which is slightly different of the ideal planar ring of 720°.It may be for the reason of the strong electrondrawing CF3group lead to the boron atom a little deviate from planar triangle sp2configuration[18].
2.3 Thermalpropertyof LAl[OB(2-CF3C6H 4)]2(μ-O)
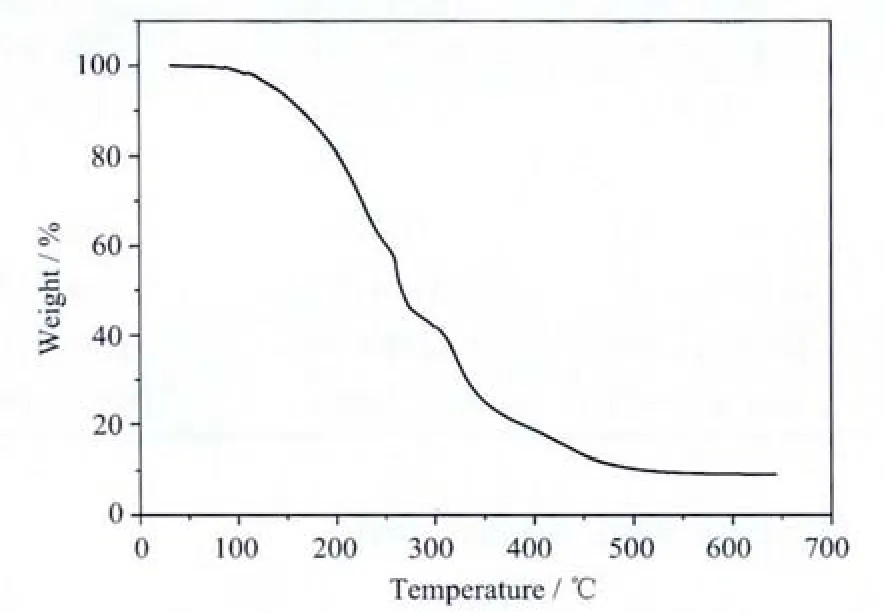
Fig.2 TGA curve of title compound
The thermal decomposition of the aluminum complex is shown in Fig.2.The initial weight loss occurs in the range of 112.6 to 550.0℃.And the TG curve shows that the weight loss corresponding to this temperature range is 91.2%that roughly coincides with the value of 91.6%.Theβ-diketiminato ligand and two 2-trifluoromethylphenyls are missing from the molecule of the complex during the course of decomposition.At last,the B2O3is formed.In conclusion,the thermo gravimetric analysis shows that the organic aluminium complex is stable below the temperature of 112.6℃.
[1]Colclough R O,Gee G,Higginson W C E,et al.J.Polym.Sci.,1959,34(127):171-179
[2]Vandenberg E J.J.Polym.Sci.,1960,47(149):486-489
[3]Colclough R O,Gee G,Jagger A H.J.Polym.Sci.,1960,48(150):273-278
[4]Ishida SI.J.Polym.Sci.,1962,62(173):1-14
[5]Saegusa T,Fujii Y,Fujii H,et al.Makromol.Chem.,1962,55(1):232-235
[6]Sinn H,Kaminsky W,Vollmer H J,et al.Angew.Chem.,Int.Ed.,1980,19(5):390-392
[7]Sinn H,Kaminsky W.Adv.Organomet.Chem.,1980,18,99-149
[8]Montero M L,Voigt,A,Teichert M,et al.Angew.Chem.,Int.Ed.,1995,34(22):2504-2506
[9]DUAN Fang-Zheng(段芳正),LI Ji-Yang(李激扬),DING Hong(丁红),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2008,24(8):1343-1346
[10]LI Li(李丽),TANG Yong-Zheng(汤勇铮),LI Hui-Hui(李卉卉),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2013,29(1):111-122
[11]LI Li(李丽),TANG Yong-Zheng(汤勇铮),XIE Yan-Fang(谢艳芳),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2010,26(6):931-938
[12]QIAN Feng(钱峰),LIU Ke-Yin(刘克印),MA Hai-Yan(马海燕).Chinese J.Catal.(Cuihua Xueba o),2011,32(1):189-196
[13]Brule E,Guo J,Coates G W,et al.Macromol.Rapid Commun.,2011,32(2):169-185
[14]Dunn E W,Coates G W.J.Am.Chem.Soc.,2010,132(33):11412-11413
[15]Clark T J,Robertson N J,Kostalik H A,et al.J.Am.Chem.Soc.,2009,131(36):12888-12889
[16]Zelikoff A L,Kopilov J,Goldberg I,et al.Chem.Commun.,2009,(44):6804-6806
[17]Korich A L,Iovine P M.Dalton Trans.,2010,39(6):1423-1431
[18]Yang Z,Ma X,Oswald R B,et al.J.Am.Chem.Soc.,2006,128(38):12406-12407
[19]Ma X L,Yang Z,Wang X,et al.Inorg.Chem.,2011,50(5):2010-2014
[20]Yang Z,Hao P,Liu Z,et al.Organometallics,2012,31(17):6500-6503
[21]Hao P,Yang Z,Ma X,et al.Dalton Trans.,2012,41(43):13520-13524
[22]Qian B,Ward D L,Smith III M R.Organometallics,1998,17(14):3070-3076
[23]Cui C,Roesky H W,Hao H J,et al.Angew.Chem.,Int.Ed.,2000,39(10):1815-1817
[24]Sheldrick G M.SADABS,Empirical Absorption Correction Program,University of Goettingen,Goettingen,Germany,1997.
[25]Sheldrick G M.Acta Crystallogr.,Sect.A,1990,46(6):467-473
[26]Sheldrick G M.SHELXL-97,Program for Crystal Structures Refinement,University of Goettingen,Goettingen,Germany,1997.
[27]Bai G,Peng Y,Roesky H W,et al.Angew.Chem.,Int.Ed.,2003,42(10):1132-1135