3,6-双硝基胍基-1,2,4,5-四嗪(DNGTz)及其衍生物的合成、表征及热性能
霍 欢,王伯周,罗义芬,樊学忠,李吉祯,周 诚,安 亭
(西安近代化学研究所,西安 710065)
0 Introduction
Recently,thenitrogen-rich energeticcompounds have been paid much more attention owing to their unique characteristics,such as high density and high specific volume[1-3].There are a large number of C—N and N—N bonds in these compounds,whereas nitrogen-rich compounds containing C—N and N—N bonds bring about the increase in density and/or specific volume for energetic compounds.Among them,tetrazine compound is a kind of interesting high energy material because of its high nitrogen content and aromaticity.3,6-Dinitroguanidino-1,2,4,5-tetrazine(DNGTz)and 3,6-dinitroguanidino-1,2,4,5-tetrazine bistriamino-guanidnium salt(TGA-DNGTz)were firstly synthesized by David E.Chavez in Los Alamos national laboratory[4].Furthermore, the performances of DNGTz and TGA-DNGTz were studied,the data exhibited that DNGTz has an exceptionally low pressure exponent of 0.163,the lowest pressure dependence known in the literature for a monopropellant[5].These compounds could be applied to some insensitive high explosives,low signal propellants,gas-forming agents and pyrotechnics[6-10].In order to continue the search for novel energetic nitrogen-rich tetrazine derivatives and decrease the acidity of DNGTz,whose acidity may lead to unacceptable safety,a new energetic compound,3,6-dinitroguanidino-1,2,4,5-tetrazine bisguanylureaium salt(M-DNGTz)were designed and synthesized from DNGTz through the reaction of neutralization and metathesis.The structures for DNGTz,TGA-DNGTz and M-DNGTz were characterized by FT-IR,1HNMR,13CNMR and elemental analysis.The optimal conditions for the reactions of oxidation and substitution were thoroughly studied.The thermal properties of three compounds,DNGTz,TGA-DNGTz and M-DNGTz,were studied by DSC and TG-DTG techniques.
1 Experiment
1.1 General methods and materials
The synthetic routes of DNGTz,TGA-DNGTz and MDNGTz is shown in Fig.1.
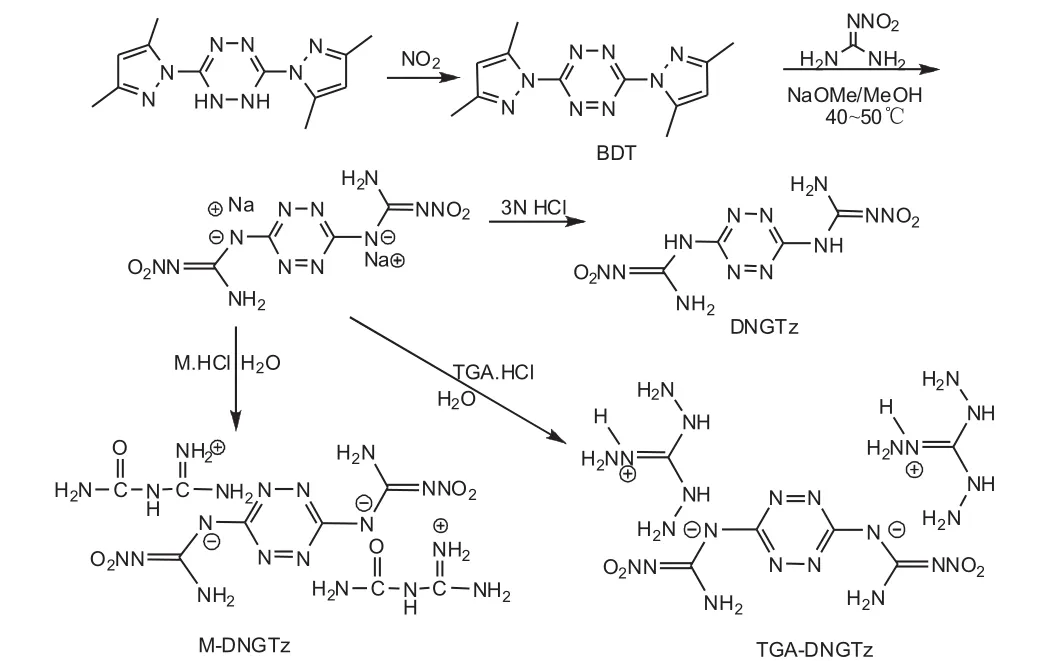
Fig.1 The synthetic routes of DNGTz,TGA-DNGTz and M-DNGTz
Melting point was determined using an open capillary tube.The IR spectrum was recorded with a NEXUS870-based Fourier infrared spectrometer employing a KBr matrix.1H NMR and13C NMR spectra were recorded with an AV500-type(500 MHz)superconducting NMR instrument.DMSO-d6and tetramethyl silane(TMS)were the solvent and an internal standard,respectively.Elemental analysis was performed on a Vario EL-ⅢElemental Analyzer.Differential scanning calorimetry(DSC)was carried out in a platinum sample container using a Shimadzu DSC-60.The 1.6 ~2.0 mg sample was heated at a rate of 10 ℃ /min.TG-DTG measurement was carried out using TGA instruments,Model TA2950 under flowing N2gas with a heating rate of 10.0 ℃ /min with samples weighting from 1.0 mg to 2.0 mg.The product purity was recorded on a LC-2010A ht liquid chromatographer.
Sodium nitrite,methanol,concentrated hydrochloric acid,nitroguanidine and N-methyl-2-pyrro-lidone(NMP)were all of A.R grade reagents;28%Sodium methoxide was C.P grade reagents;triamino-guanidine nitrate[11],guanylurea hydrochloride[12]and 3,6-bis(3,5-dimethylpyrazole-1-yl)-1,2-di-hydro-1,2,4,5-tetrazine[13]were prepared in our laboratory.
1.2 Synthesis of 3,6-bis(3,5-dimethylpyrazole)-1,2,4,5-tetrazine(BDT)
3,6-bis(3,5-dimethylpyrazole-1-yl)-1,2-dihydro-1,2,4,5-tetrazine(14 g,51.5 mmol),200 ml NMP were mixed and stirred.To the reaction mixture,the new preparation of NO2,which was obtained by the reaction of sodium nitrite(41.4 g,600 mmol)with 64 mL of concentrated hydrochloric acid(720 mmol),were bubbled.After stirring for 4 ~ 5 h,the flask was sealed off,and stirred for another 12 h.The red precipitate was filtered to obtain 13.5 g solid with a yield of 97.0%,and the filtrate was collected to reuse in next reaction.IR(KBr)υ:3 405,3 084,2 421,1 682,1 577,1 405,1 029,939 cm-1;1H NMR(CD3Cl,500 MHz)δ:6.18(2H,CH),2.77(6H,NCCH3),2.43(6H,N=CCH3);Anal.Calcd for C12N8H12:N 42.89,C 53.66,H 5.282,found N 41.46,C 53.32,H 5.22.
1.3 Synthesis of 3,6-dinitroguanidino-1,2,4,5-tetrazine(DNGTz)
Nitroguanidine(8.1 g,111 mmol),28%sodium methoxide(15.1 ml,111 mmol)and 200 ml methanol were added in a three-necked round-bottomed flask with a stirrer.To the reaction mixture,BDT(10.0 g,31.6 mmol)was added in batches.After BDT was added completely,the mixture was stirred for another 4 h at 45℃.The red precipitate was filtered to obtain 9.85 g solid with a yield of 95.7%.IR(KBr)υ:1 339,1 555(NO2),1 635(C=N),3 199(NH),3 451,3 364(NH2)cm-1;1HNMR(DMSO-d6,500 Hz)δ:12.236(2H,s,N—H),9.737 ~ 9.368(4H,d,J=184.5,NH2);13CNMR(DMSO-d6,500 Hz)δ:158.803,157.193;A-nal.Calcd for C4N12H6O4:C 16.78,N 58.74,H 2.098,found C 17.01,N 58.23,H 2.104.
1.4 Synthesis of 3,6-dinitroguanidino-1,2,4,5-tetrazine bistriaminoguanidnium salt(TGA-DNGTz)
3,6-dinitroguanidino-1,2,4,5-tetrazine disodium salt(5.0 g,15.2 mmol)was transferred into a three-necked round-bottomed flask with a mechanical stirrer,and 50 ml distilled water was added at room temperature.After triaminoguanidine nitrate(6.0 g,36.6 mmol)was added,the reaction mixture was stirred for 3 h at 45℃,and then cooled down to 0℃.The red precipitate was filtered,washed with ice water and finally dried to obtain 6.79 g with a yield of 90.6%.IR(KBr)υ:1 366,1 520(NO2),1 643(C=N),3 365,3 320(NH2),3 253(NH)cm-1;13CNMR(DMSO-d6,500Hz)δ:162.764,160.521,159.035;Anal.Calcd for C6N24H22O4:C 14.58,N 67.99,H 4.49,found C 14.53,N 67.86,H 4.547.
1.5 Synthesis of 3,6-dinitroguanidino-1,2,4,5-tetrazine bisguanylureaium salt(M-DNGTz)
3,6-dinitroguanidino-1,2,4,5-tetrazine disodium salt(5.0 g,15.2 mmol)was transferred into a three-necked round-bottomed flask with a mechanical stirrer,and then 50 ml distilled water was added at room temperature.After guanylurea hydrochloride(5.0 g,36.1 mmol)was added and the reaction mixture was stirred for 3 h at 45℃,then cooled down to 0℃.The red precipitate was filtered,washed with ice water and finally dried to obtain 7.0 g with a yield of 90.4%.IR(KBr)υ:1 360,1 563(NO2),1 600(C=N),1 719(C=O),3 366,3 589(NH2),3 188(NH)cm-1;Anal.Calcd for C8N20H18O6:C 19.68,N 53.09,H 3.778,found C 19.59,N 57.14,H 3.673.
2 Results and discussion
2.1 Effect of NMP recycling times on oxidation reaction
In the preparation of BDT from the oxidation reaction,NMP was selected as a common reaction solvent in order to obtain BDT in a good purity which could be used in the next substitution reaction.NMP was an expensive reagent and a mass of NMP was used up in the oxidation reaction to result in that its cost of preparation is higher.Our research group had tried to replace NMP by other reagents solvents,but the purity of obtained BDT was lower to not satisfy with the needs of next reaction.In order to reduce its costs,the reuse and recycling times of NMP were studied listed in Table 1.The data is shown as follows:The oxidation reaction might produce a small amount of water to result in that the yield and purity of the next reaction were reduced.If the recycling times of NMP was in excess of 3 times,the yield of BDT was notability reduced,therefore the 3 recycling times was adopted in the preparation of BDT.

Table 1 Effect of NMP recycling times on reaction
2.2 Effect of various factors on substitution reaction
In the substitution reaction,the various factors,such as reaction time,temperature and mole ratio are listed on Table 2~Table 4.
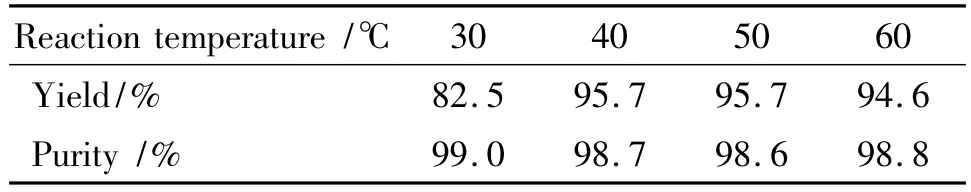
Table 2 Effect of temperature on reaction1)
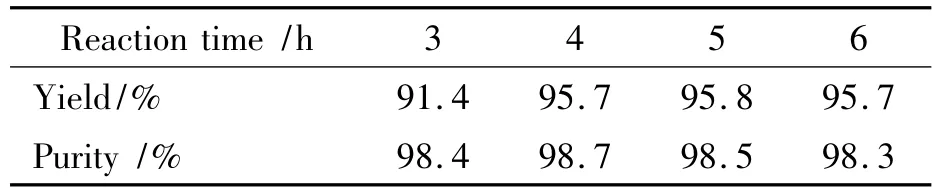
Table 3 Effect of time on reaction1)
It is found from Table 2 that the yield of BDT is 82.5%at the reaction temperature of 30 ℃ ,belonging to incomplete reaction;At reaction temperature of 40~50℃,the yields of DNGTz were over 95%;The reaction temperature was sequentially increased,and its yield was not increased anymore.Thus,the optimal reaction temperature was 40~50℃.
It is found from Table 3 that the reaction is incomplete at reaction time of 3 h with a yield of 91.4%and purity of 98.4%;At reaction time of 4 h,DNGTz was synthesized with a yield of 95.7% and a purity of 98.7%;Its yield was not increased any more if the reaction time was sequentially prolonged,therefore,the optimal reaction time was 4 h.
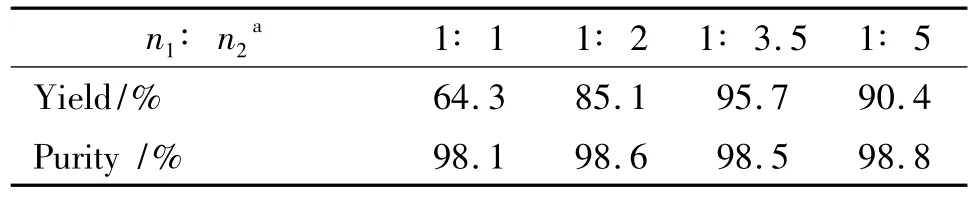
Table 4 Effect of BDT and sodium methoxide mole ratio on reaction1)
It is showed from Table 4 that molar ratio of BDT and sodium methoxide is a key factor in this reduction.Using n(BDT):n(sodium methoxide)as 1∶1,DNGTz was synthesized with a yield of 64.3%,the reduction was incomplete because BDT had been detected by1HNMR,13CNMR and elemental analysis;Using molar ratio as 1∶3.5,the highest yield of DNGTz was reached to 95.7%with a purity of 98.5%;The molar ratio of BDT and sodium methoxide was sequentially increased,and its yield was not further increased.Thus,the optimal molar ratio was 1∶3.5.
2.3 Physical chemistry and detonation properties of DNGTz,M-DNGTz and TGA-DNGTz
These densities and decomposition temperature are obtained by the tests,and the other performances are obtained by calculation,in which detonation properties were calculated by Gaussian 98 program[14].The results is listed in Table 5.It is found that TGA-DNGTz has a better performances with the nitrogen content of 63.2%and detonation velocity of 9 544.9 m/s,and it may be a potential oxidizer to be applied in propellants.
2.4 Thermal behaviors of DNGTz,M-DNGTz and TGA-DNGTz
The thermal behaviors of DNGTz,M-DNGTz and TGA-DNGTz are studied by DSC and TG-DTG with heating rate of 10.0 ℃ /min,and the DSC,TG-DTG curves of the three energetic materials are shown in Fig.2 ~ Fig.7.
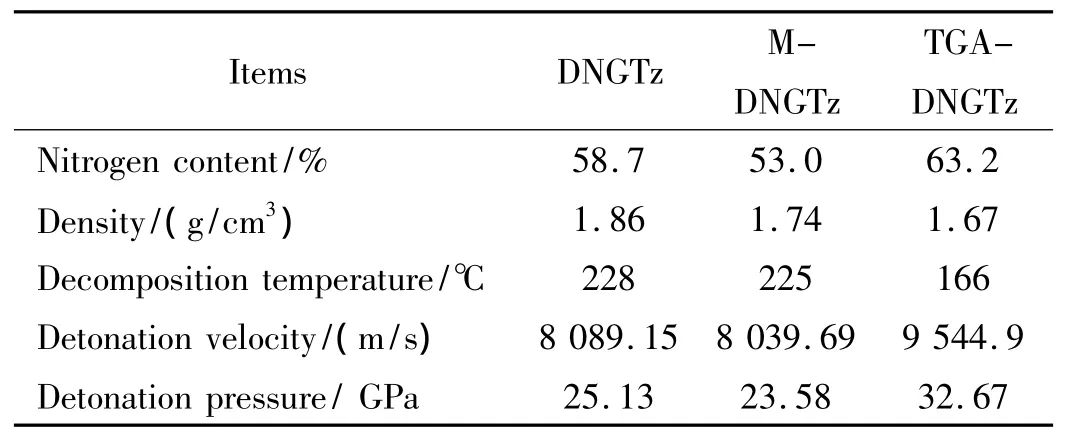
Table 5 The performances of physico-chemistry and detonation for DNGTz,M-DNGTz and TGA-DNGTz
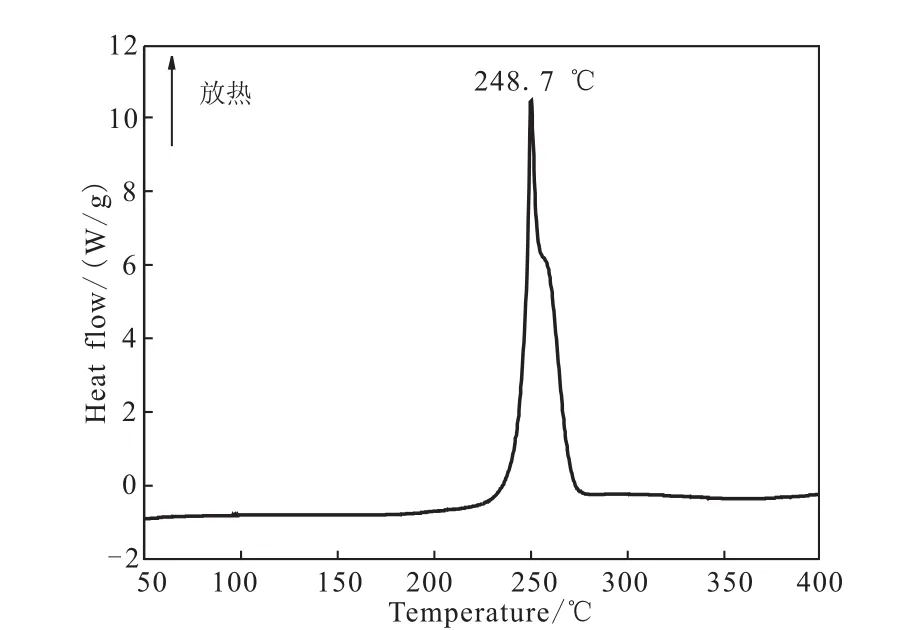
Fig.2 DSC curve of DNGTz
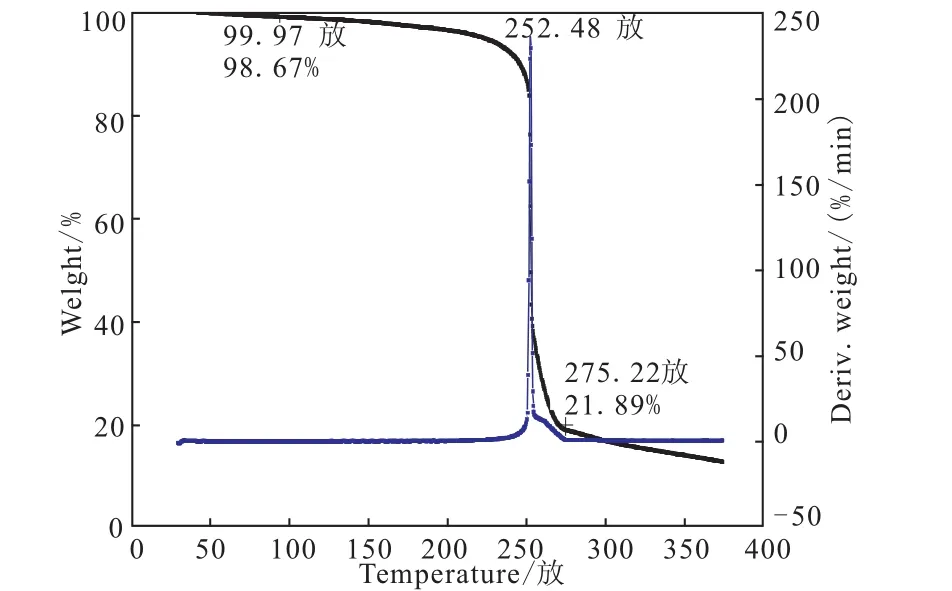
Fig.3 TG-DTG curves of DNGTz
The DSC and TG-DTG analysis of DNGTz reveal that it is thermally stable up to 248.7 ℃.The DSC curve of DNGTz in Fig.2 exhibit a thermal decomposition peaks at 248.7 ℃ according to the one obvious mass-loss stages in the TG-DTG curves in Fig.3,which can be confirmed by the mass-loss stage in the temperature range of 99.97 ~275.22 ℃ with mass-loss of 76.78%.
The DSC curve of M-DNGTz in Fig.4 exhibit a melting point,Tmax,at 208.7℃,and two thermal decomposition peaks at 212.7℃ and 248.2℃,respectively.The TG-DTG curve of M-DNGTz in Fig.5 show four main mass loss stages.The first stage amounts to 7.27% in the range of 58.9 ~84.44 ℃,it is mainly attributed to the part of guanylurea hydrochloride.The second stage begins at 133℃ and ends at 143.95℃,accompanied with 4.26%mass loss,corresponding to the mass of residual guanylurea hydrochloride.The third stage amounts to 17.2%in the range of 203.32 ~228.70 ℃,which is in agreement with the mass fraction amount DNGTz,corresponding to the obvious peak in DSC curve in the temperature range.The fourth stage occurs with a weight loss of 46.31%in the range of 228.7 ~285.77 ℃,corresponding to the mass of DNGTz.and 15.58% residue at 497.95℃,which indicates that there are a few remains at the end of the decomposition.
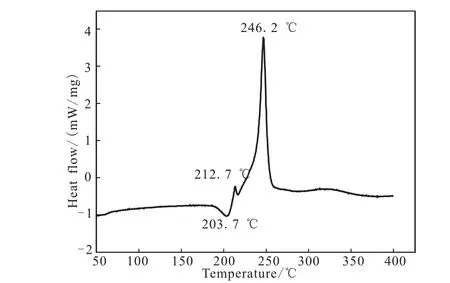
Fig.4 DSC curves of DNGTz
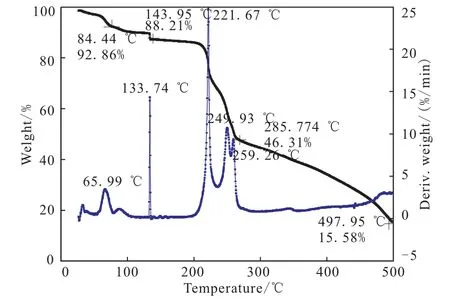
Fig.5 TG-DTG curves of MDNGTz
The DSC curve of TGA-DNGTz in Fig.6 exhibit a melting point,Tmax,at 88.74,and one thermal decomposition peaks at 180.2 ℃.In the TG-DTG curve of Fig.7,TGA-DNGTz show three stages of decomposition.The first stage amounts to 19.21%in the range of 49.1 ~105.89℃,corresponding to the mass of triaminoguanidine.The second stage begins at 182.01 ℃ and ends 200.50℃,it is mainly attributed to the decomposition of DNGTz,corresponding to the obvious peak in DSC curve in the temperature range.In the temperature range of 242.48 ~265.97℃,the mass loss is less than 10%,which indicates that a few remains at the end of the decomposition,and 22.23%residue at 491.13 ℃.
The above-mentioned DSC and TGA results show that their first decomposition temperatures are 248.7 ℃,212.7 ℃,190.2 ℃,respectively.The fact that their decomposition temperatures are over 190℃show that they exhibit excellent thermal stability and they might be potentially useful as gas-forming agents or rocket propellants.
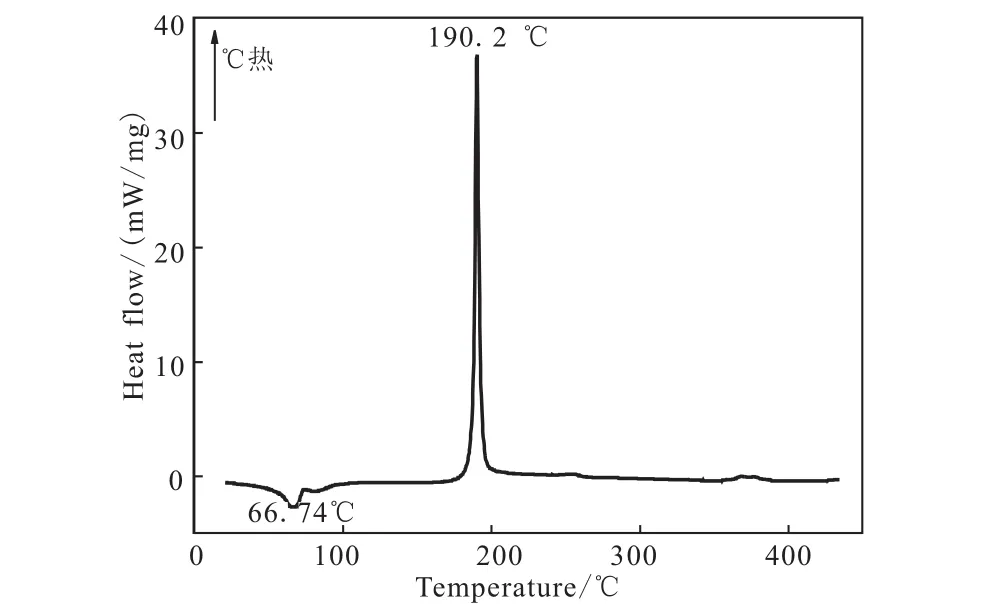
Fig.6 DSC curve of TGA-DNGTz
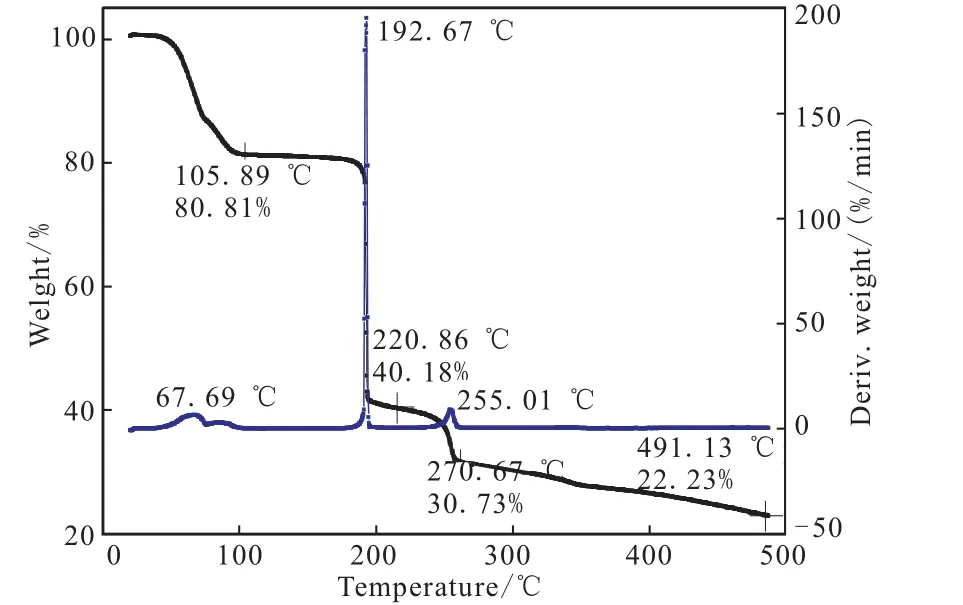
Fig.7 TG-DTG curve of TGA-DNGTz
2.5 Reaction mechanism of substitution
It may be concluded from carbon of tetrazine ring with 3,5-dimethylpyrazolyl in BDT molecule that carbocations were formed easily,and attacked by nucleophilic nitroguanidine.In this way,intermediate(I)was obtained,then in alkaline medium,3,6-dinitroguanidino-1,2,4,5-tetrazine disodium salt was produced through 3,5-dimethylpyrazole group drawing off,finally acidified by hydrochloric acid to obtain the aimed compound DNGTz,its assumed reaction mechanism is shown in Fig.8.
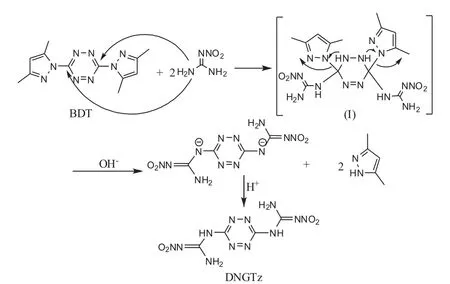
Fig.8 Mechanism of substitution for synthesis of DNGTz
3 Conclusions
3,6-Dinitroguanidino-1,2,4,5-tetrazine(DNGTz)is synthesized with a total yield of 92.8%,and its derivative 3,6-dinitroguanidino-1,2,4,5-tetrazinebistriaminoguanidnium salt(TGA-DNGTz)is synthesized by the literature method.A new energetic compound 3,6-dinitroguanidino-1,2,4,5-tetrazine bisguanylureaium salt(M-DNGTz)derived from DNGTz are designed,synthesized and characterized in the first.It is experimentally found that the conditions of substitution reaction are optimized as:the reaction time is 4 h,the reaction temperature is 40~50℃,BDT and sodium methoxide mole ratio is 1∶3.5.The performances of physico-chemistry and detonation,thermal behaviors for DNGTz,TGA-DNGTz and M-DNGTz are studied,their detonation velocities are found to be 8 089.15 m/s,9 544.9 m/s,8 039.69 m/s,and their decomposition temperatures are 248.7 ℃,190.2 ℃,246.2 ℃,respectively.The fact which their detonation velocities are over 8 000 m/s,and decomposition temperatures are over 190℃,show that the three energetic compounds exhibit excellent thermal stability and detonation properties.
Reference:
[1]Jochen K,Stefan L.Synthesis and characterization of 3,3-'azobis(6-amino-1,2,4,5-tetrazine)DAAT-a new promising nitrogen-rich compound[J].Propellants,Explosives,Pyrotechnics,2002,27:111-118.
[2]Churakov A M,Smirnov O Y,Loffe S L.Benzo-1,2,3,4-tetrazine 1,3-Dioxides:Synthesis and NMR Study[J].Eur.J.Org.Chem.2002:2342-2349.
[3]Hiskey M A,Chavez D E,Son S F,et a1.Progress in highnitrogen chemistry in explosives,propellants and pyrotechnics[C]//Proc.27th International Pyrotechnics Seminar July 16-21,USA:Colorado,2000:3-14.
[4]Pan J,He J X,Tao Y J.Synthesis and characterization of 3,6-dihydrazine-1,2,4,5-tetrazine and its energetic salt[J].Chin.J.Energ.Mater.,2006,14:116-117.
[5]Xu S L,Yang,S Q,Yue S T.Synthesis and research of 3,6-bis(1H-1,2,4-tetrazol-5-yl-amino)-1,2,4,5-tetrazine[J].Chin.J.Energ.Mater.,2004,12:44-47.
[6]Chavez D E,Hiskey M A,Gilard R D.Novel high-nitrogen materials based on nitroguanyl-substituted tetrazines[J].Organic Letters,2004,6(17):2889-2891.
[7]Chavez D E,Tappan B C,Hiskey M A,et a1.New high-nitrogen materials based on nitroguanyl-tetrazines:explosive properties,themal decomposition and combustion studies[J].Propellants,Explosives,Pyrotechnics,2005,30:412-417.
[8]Nurullah Saracoglu.Recent advances and application in 1,2,4,5-tetrazine chemistry[J].Tetrahedron,2007,63:4199-4236.
[9]Chavez D E,Hiskey M A,Naud D L.Tetrazine explosives[J].Propellants,Explosives,Pyrotechnics,2004,29:209-215.
[10]Pan J,He J X,Tao Y J Chin.Synthesis and characterization of 3,6-diamine-1,2,4,5-tetrazine[J].J.Energ.Mater,2004,12:58-59.
[11]Wang B Z,Lai W P,Liu Q Synthesis,Characterization and quantum chemistry study on 3,6-Bis(1H-1,2,3,4-tetrazol-5-yl-amino)-1,2,4,5-tetrazine[J]. Chin. J.Org.Chem.,2008,28:422-427.
[12]Liu Q,Wang B Z,Zhang Z Z.Synthesis and properties of N-guanylurea Dinitramide[J].Chinese Journal of Explosives and Propellants.2006,29:29-31.
[13]Wang B Z,Liang P Liu,Q Zhang H H,et al.Synthesis improvement of 3,6-diguanidino-1,2,4,5-tetrazine and its salt[J].Chin.J.Energ.Mater,2006,14:352-354.
[14]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 09,Gaussian,Inc.[R].Wallingford CT,2009.

