Performance of Ni-based, Fe-based and Co-based Oxygen Carriers in Chemical-Looping Hydrogen Generation
Liang Hao; Zhang Xiwen; Fang Xiangchen; Yuan Honggang
(1. Fushun Research Institute of Petroleum and Petrochemicals, SINOPEC, Fushun 113001; 2. Fushun Research Institute of Environmental Science, Fushun 113006)
Performance of Ni-based, Fe-based and Co-based Oxygen Carriers in Chemical-Looping Hydrogen Generation
Liang Hao1; Zhang Xiwen1; Fang Xiangchen1; Yuan Honggang2
(1. Fushun Research Institute of Petroleum and Petrochemicals, SINOPEC, Fushun 113001; 2. Fushun Research Institute of Environmental Science, Fushun 113006)
Ni-based, Fe-based and Co-based oxygen carriers with perovskite oxides used as the supports were prepared by citric acid complexation method. The oxygen carriers were characterized by thermal analysis, H2-temperature-programmed reduction and X-ray diffraction methods. Performance tests were evaluated through Chemical-Looping Hydrogen Generation in a fixed-bed reactor operating at atmospheric pressure. The characterization results showed that all samples were composed of metal oxides and perovskite oxides. Performance results indicated that CH4conversion over the oxygen carriers decreased in the following order: NiO/LaNiO3>Co2O3/LaCoO3>Fe2O3/LaFeO3. The ability of NiO/LaNiO3and Fe2O3/ LaFeO3to decompose water was stronger than that of Co2O3/LaCoO3as evidenced by our experiments. H2amounting to 80 mL upon reacting on methane in every cycle could be completely oxidized by NiO/LaNiO3at 900 ℃ in the period from the third cycle to the eighth cycle.
Chemical-Looping Hydrogen Generation; oxygen carrier; perovskite oxide; methane
1 Introduction
The current worldwide hydrogen demand is predominantly fulfilled by the steam methane reforming process on a large scale[1]. Water gas shift (WGS) reaction is needed after steam methane reforming process[2-3]. Therefore CO2is formed with H2in outlet gases simultaneously. CO2separation is generally realized by pressure swing adsorption process (PSA), which would consume a large amount of energy.
The Chemical-Looping Hydrogen Generation (abbreviated hereinafter as CLHG) process is a novel method for hydrogen generation (as presented in Figure 1)[4-5]. CLHG with the advantage of inherent CO2separation attracts the attention of many researchers. This hydrogen production method is different from the chemical-looping methane reforming process for hydrogen generation. CLHG is divided into two reactions that are carried out in two reactors, respectively. The mechanism of the process is based on the chemical-looping combustion process. Firstly, the oxygen carrier is reduced by fuel in the fuel reactor, and gases exiting the fuel reactor are composed of CO2and steam[6]. All the carbon dioxide gas is gathered by condensation of steam and this process requires less energy consumption[7]. The reduced oxygen carrier is then transferred to the steam reactor where it is oxidized by steam. H2is the product resulted from decomposition of steam in the presence of the reduced oxygen carrier. The whole process is a complete redox cycle. And then the oxygen carrier undergoes the whole chemical cycle by passing through the fuel reactor and the steam reactor in turn.
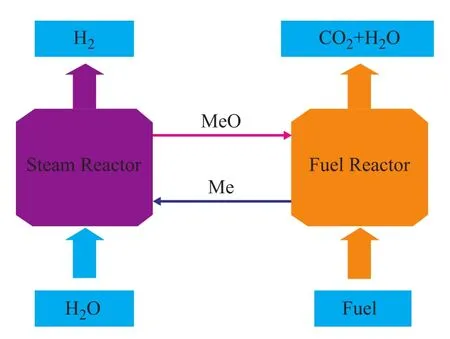
Figure 1 Simplified diagram of CLHG process
The use of metal oxides in cyclic redox reactions to produce H2is not new[8-9]. Iron ores have been used for thispurpose in the steam-iron process dating almost a century back. Most of the designs were tailored to produce H2since CO2separation was not considered essential at that time. At present, the outlet gas treatment was necessary. Oxygen carrier is the key factor for realizing the CLHG process. It must have strong ability to oxidize fuels, and the reduced oxygen carrier must have excellent ability to decompose steam[9]. Therefore oxygen atoms in the oxygen carrier must be active whether in reduction process or in oxidization process. Usually, the CLHG process is generally realized at high temperature owing to the highly endothermic nature of the process[10]. It is well known that perovskite oxides are stable during high-temperature reactions, and structural change of perovskite oxides can lead to the formation of oxygen vacancies and increase mobility of oxygen atoms caused by changes in crystal lattice[11-12]. As far as the issue is concerned, literature information about CLHG is not adequate, with Fe2O3only being referred to on its use in the CLHG process[4]. Up to now there is less literature referring to the application of perovskite oxide as the support in the CLHG process.
The purpose of this study is to discuss the performance of perovskite oxides, which are applied as the support of Nibased, Fe-based and Co-based oxygen carriers for realizing the CLHG process, and to afford reliable data for the application of perovskite oxides in the CLHG technology.
2 Experimental
2.1 Preparation of oxygen carriers
The perovskite oxides were prepared by the citric acid complexation method. The reagents such as Ni(NO3)2·6H2O (AR), Fe(NO3)3·9H2O (AR), Co(NO3)2·6H2O (AR) and La(NO3)3·6H2O (AR) were used as metal sources. A solution containing 15 m% of NiO/LaNiO3was prepared according to the following procedure: 36.3 g of Ni(NO3)2·6H2O and 34.3 g of La(NO3)3·6H2O were dissolved in 100 mL of water, to which a citric acid solution was added dropwise at 40 ℃. After a while, the temperature was increased to 80 ℃ which was maintained until the solution became a gel. The gel was dried at 110 ℃ for 48 h in a drying oven, and was then calcined at 900 ℃ for 4 h in a muffle furnace.
Aqueous solutions composed of 15 m% of Fe2O3/LaFeO3and 15 m% of Co2O3/LaCoO3, respectively, were prepared according to the same preparation procedure as mentioned above.
2.2 Characterization techniques
For the identification of crystalline phases, powder X-ray diffraction (XRD) patterns were acquired by a diffractometer D/Max-2500 using a Ni-filtered Cu Kα radiation. The temperature-programmed reduction (TPR) profiles were obtained by passing through 100 mg of sample under a gas containing 10 vol % of H2in Ar stream flowing at a flowrate of 100 cm3/min. The thermo-gravimetric analysis was carried out in a DSC204HP TG instrument under a dry-air stream at a heating rate of 10 ℃/min.
2.3 Performance tests
The performance of oxygen carriers was studied in a fixed-bed quartz reactor (0.8 cm in inside diameter and 60 cm in height) at 900 ℃ and under atmospheric pressure. The fuel was a gas mixture consisting of pure methane with a flow rate of 10 mL/min and N2with a flow rate of 90 mL/min. The sample amount was 1.24 g. The amount of water was 0.5 mL added at a flow rate of 0.2 mL/min. Prior to the reaction tests, oxygen carriers were calcined in situ in dry air (at a rate of 30 mL/min) for 1 h at 300 ℃with the oven reaching the required reaction temperature subsequently. During the reduction process, CH4and N2passed over the oxygen carrier at 900 ℃ for three minutes. Then N2was introduced in order to purge out methane remaining in the reactor. Water vapor diluted with N2was passed over the reduced Ni-based oxygen carrier at 900 ℃ for five minutes. Then N2was introduced in order to purge out steam remaining in the reactor. Then air was introduced in order to oxidize the Ni-based oxygen carrier completely for five minutes. The reduction process with methane and the subsequent oxidation process with water vapor and air were repeated under the same conditions. The inlet and outlet gases from the reactor were analyzed by a gas chromatograph equipped with a packed column, a porapak Q column, and a thermal conductivity detector.
3 Results and Discussions
3.1 Thermal analysis
Figure 2 shows the TG-DSC curves of Fe2O3/LaFeO3,Co2O3/LaCoO3and NiO/LaNiO3, respectively. It can be seen from Figure 2(a) that the mass loss before 160 ℃ is ascribed to the evaporation of adsorbed water and structural water, and the sharp mass loss between 160 ℃ and 330 ℃is caused by the decomposition of NO3-species, while the mass loss in the temperature range of 330 ℃—360 ℃ is ascribed to the combustion of superfluous citric acid complexation, which is a strongly exothermic process, and the mass loss after 450 ℃ is attributed to the decomposition of La2O2CO3. The TG-DSC curves of Co2O3/LaCoO3and NiO/LaNiO3are similar to those of Fe2O3/LaFeO3, which are not addressed further. In one word, the final calcination temperature is specified at 900 ℃ for all samples.
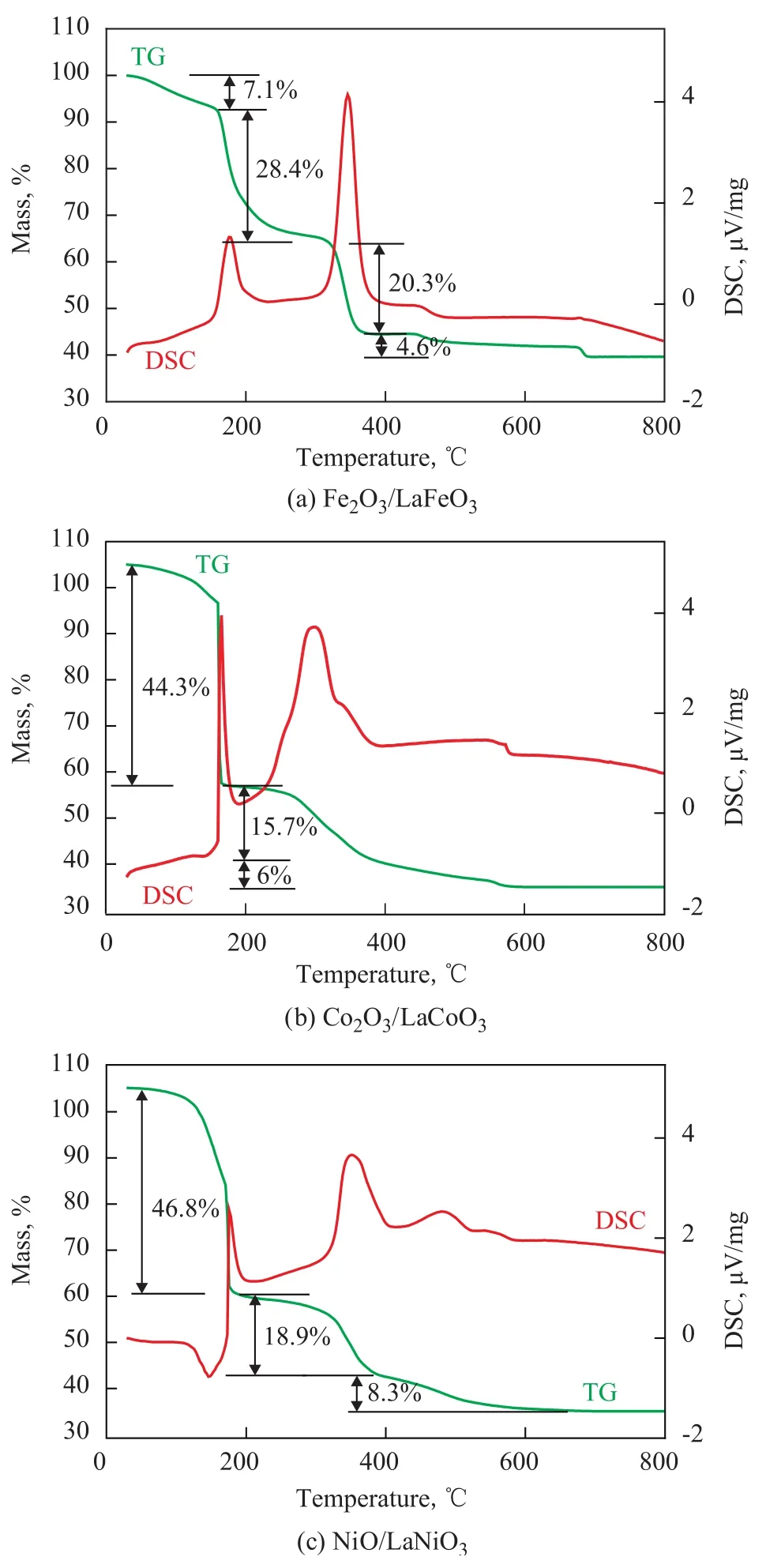
Figure 2 Thermal analysis of oxygen carriers
3.2 X-ray diffraction
The XRD patterns of Fe2O3/LaFeO3, Co2O3/LaCoO3and NiO/LaNiO3are shown in Figure 3. The diffraction peaks of perovskite structure can be observed in all oxygen carriers. Besides, the diffraction peaks of Fe2O3, Fe3O4, NiO and Co2O3are identified in Figure 3 (a), Figure 3 (b)and Figure 3 (c) respectively. All these diffraction peaks indicate that the oxygen carriers are composed of metal oxides and perovskite oxides.
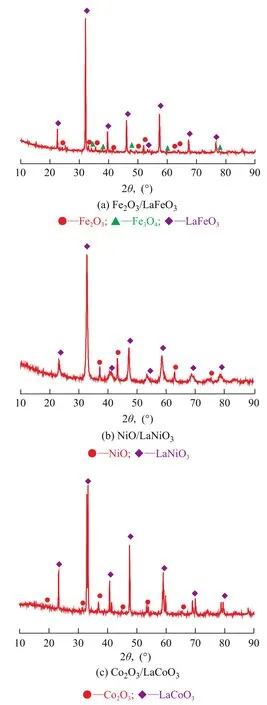
Figure 3 XRD patterns of the oxygen carriers
3.3 Temperature programmed reduction tests
Reduction properties of oxygen carriers are very critical in the redox cycle. The reduction properties of three kinds of oxygen carriers are presented in Figure 4. It can be seen from Figure 4 (a) that LaFeO3can hardly be reduced before 700 ℃, but the addition of Fe2O3to LaFeO3improves the reduction property of LaFeO3obviously, and LaFeO3can be reduced at 300℃. The big reduction peak around 500 ℃ is attributed to the reduction processes involving Fe3+→Fe0and the reduction of surface adsorbed oxygen in LaFeO3. The reduction peak which is higher than 700 ℃ is attributed to the reduction of lattice oxygen atoms in bulk LaFeO3particles.

Figure 4 H2-TPR patterns of three oxygen carriers
As to the oxygen carrier LaNiO3, there are two main reduction peaks at 300 ℃ and 480 ℃ as shown in Figure 4(b). The first one is attributed to the reduction of adsorbed oxygen, and the second one is attributed to the reduction of lattice oxygen in bulk LaNiO3particles. The addition of NiO facilitates the reduction of LaNiO3, and areas of all related peaks become much larger. There are two reduction peaks located between 200 ℃and 400 ℃. The former peak is related with the reduction process involving Ni2+→Ni0, and the latter peak is related with the reduction of adsorbed oxygen atoms. The same phenomenon occurs to LaCoO3. The addition of Co2O3to LaCoO3significantly favors the reduction of LaCoO3as shown in Figure 4(c).
All H2-TPR profiles of the oxygen carriers showed that metal oxides could improve the reduction capacity of the corresponding perovskite oxides. There are more oxygen atoms which take part in the redox process over Fe2O3/LaFeO3, Co2O3/LaCoO3and NiO/ LaNiO3than that over LaFeO3, LaCoO3and LaNiO3, respectively.
3.4 Performance evaluation
The performance evaluation tests are carried out via a two-step reaction. The first step is the reduction of oxygen carrier by CH4, and the second one is the oxidation of oxygen carrier by steam to produce H2.
Firstly, CH4conversion is evaluated over these three oxygen carriers (Figure 5). CH4conversion over the oxygen carriers decreases in the following order: NiO/LaNiO3>Co2O3/LaCoO3>Fe2O3/LaFeO3. When the temperature reaches 900 ℃, CH4is oxidized completely over both of oxygen carriers NiO/LaNiO3and Co2O3/LaCoO3, and the methane conversion rate is much higher than that over the oxygen carrier Fe2O3/ LaFeO3.
The hydrogen yield that varies with reaction temperature over the oxygen carriers is shown in Figure 6. It is found out that H2yield shows an increasing trend with the in-crease of reaction temperature. At 900 ℃, the H2yield over NiO/LaNiO3is almost the same as that over Fe2O3/ LaFeO3, while the hydrogen output over Co2O3/LaCoO3is the lowest. This behavior reveals that the ability of NiO/LaNiO3and Fe2O3/LaFeO3to decompose water is stronger than that of Co2O3/LaCoO3as evidenced by our experiments. It is well known that Ni particles cannot react upon steam according to the thermodynamic calculation, however, the above results have manifested that the oxygen carrier NiO/LaNiO3can react with steam to produce H2. It is speculated that the vacant sites of oxygen atoms, other than the Ni particles, are active sites taking part in the reaction. Steam in the steam reactor actually oxidizes the vacant sites of oxygen atoms to release H2. The composition of gases exiting the fuel reactor is given in Figure 7. It is well known that CH4can be oxidized to form CO2, CO, H2and H2O. It can be seen from Figure 7 that CO2is the major product at the start of run, but its amount decreases sharply with time, while the amount of H2and CO increases with the reaction time. This occurs because lattice oxygen atoms in perovskite oxides participate gradually in the oxidization process with the increase in reaction time, and the lattice oxygen atoms can always be involved in the partial oxidization of methane. Therefore hydrogen is produced during methane decomposition.
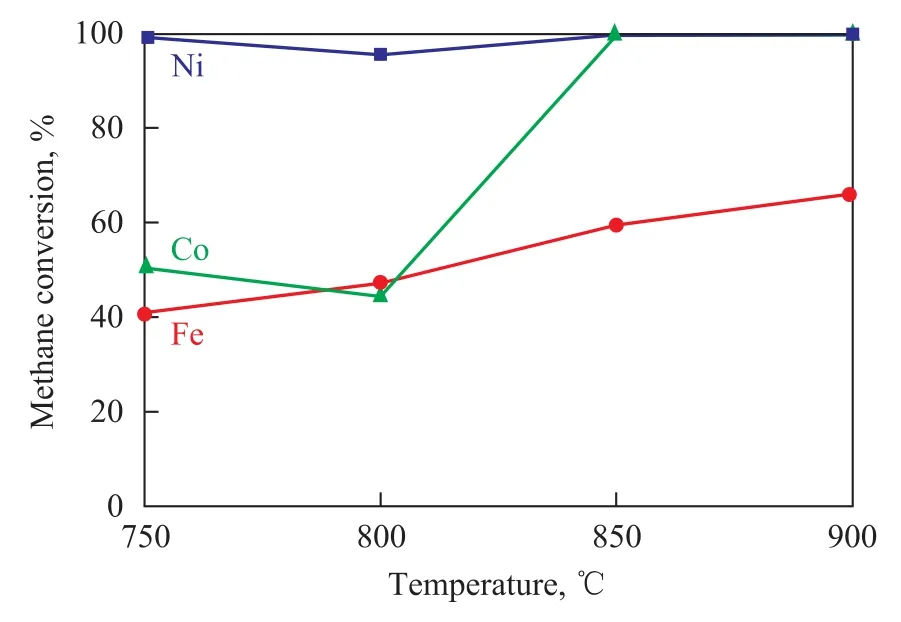
Figure 5 Variation in CH4conversion with reaction temperature over three oxygen carriers
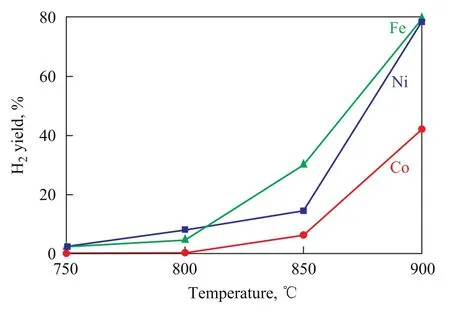
Figure 6 Hydrogen yield as function of temperature over three oxygen carriers
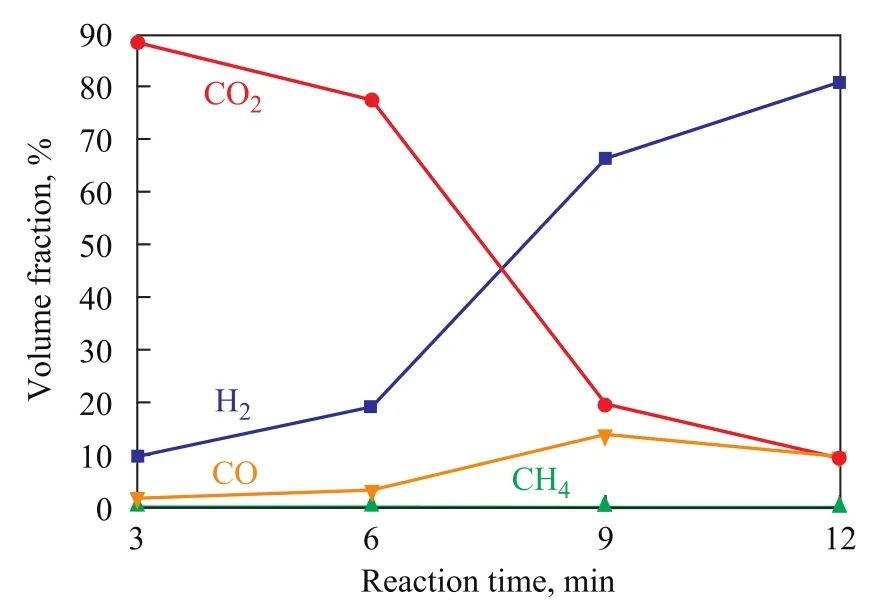
Figure 7 Effect of reaction time on the composition of outletgases produced over NiO/LaNiO3
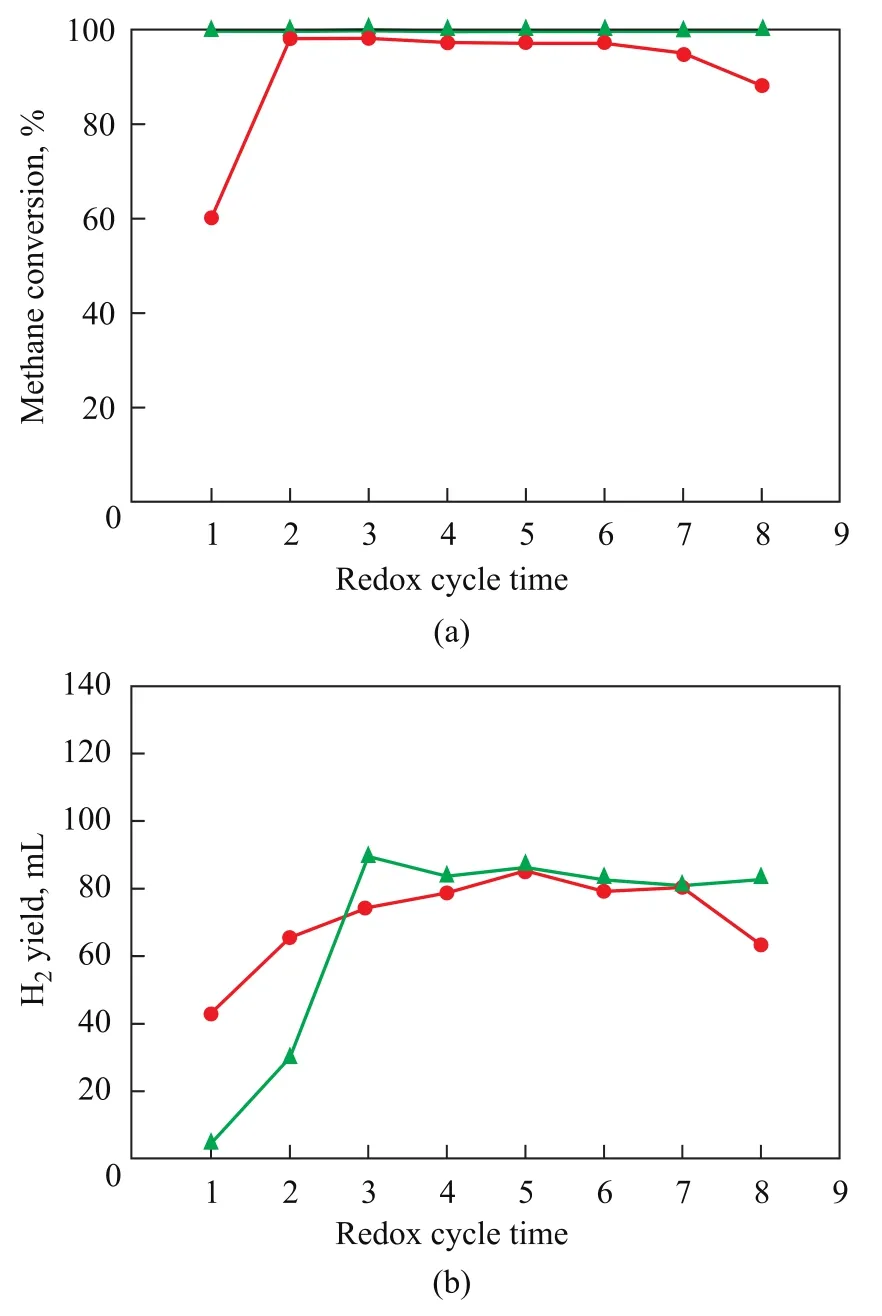
Figure 8 Comparison of oxygen carriers on their catalytic performance
Figure 8 exhibits the catalytic performance of NiO/ LaNiO3and NiO/SiO2. Figure 8 (a) shows that CH4isoxidized totally over NiO/LaNiO3and CH4is partly oxidized over NiO/SiO2in eight cycles. This result demonstrates that LaNiO3is not inert, and takes part in the oxidization process of CH4. Figure 8 (b) shows that H2output stabilizes at 80 mL starting from the fourth operating cycle over NiO/LaNiO3. However, H2output changes a lot during the eighth operating cycle over NiO/SiO2. H2output is related with the CH4conversion, the more CH4is oxidized, the more H2is formed. Though eight redox cycles are too short to manifest the catalytic stability, the role which LaNiO3plays can be obviously seen in Figure 8.
4 Conclusions
A novel process is developed for oxidizing natural gas to hydrogen. The oxygen carriers Fe2O3/LaFeO3, Co2O3/ LaCoO3, and NiO/LaNiO3were prepared by the citric acid complexation method. XRD results showed that all the samples are composed of metal oxides and perovskite oxides. The performance evaluation tests showed that the highest conversion of CH4and the maximum amount of H2were achieved over NiO/LaNiO3, which was attributed to the most reduction-prone oxygen atoms in NiO/ LaNiO3. The excellent performance of the oxygen carriers investigated thereby has revealed that CLPG of methane using NiO/LaNiO3as the oxygen carrier should be feasible for further study.
Acknowledgement:This work was financially supported by China Petrochemical Corporation (SINOPEC) (Contact No. 106002000284).
Reference
[1] Aasberg-Petersen K, Bak Hansen J H, Christensen T S, et al. Technologies for large-scale gas conversion[J]. Applied Catalysis, A: General, 2001, 221(1/2): 379-387
[2] Gideon Botes F. Water-gas-shift kinetics in the iron-based low-temperature Fischer-Tropsch synthesis[J]. Applied Catalysis, A: General, 2007, 328(2): 237-242
[3] Denkwitz Y, Karpenko A, Plzak V, et al. Influence of CO2and H2on the low-temperature water-gas shift reaction on Au/CeO2catalysts in idealized and realistic reformate[J]. Journal of Catalysis, 2007, 246 (1): 74-90
[4] Yang Jingbiao, Cai Ningsheng, Li Zhenshan. Hydrogen production from the steam-iron process with direct reduction of iron oxide by chemical looping combustion of coal char[J]. Energy & Fuel, 2008, 22 (4): 2570-2579
[5] Gupta P, Velazquez-Vargas L G, Fan Liang-Shih. Syngas redox (SGR) process to produce hydrogen from coal derived syngas[J]. Energy & Fuel, 2007, 21(5): 2900-2908
[6] Shi Q L, Chen S Y, Xue Z P, et al. Experimental investigation of chemical looping hydrogen generation using iron oxides as oxygen carrier[J]. Proceedings of the CSEE, 2011, 31(z): 168-174
[7] Mattisson T, Lyngfelt A, Cho Paul. The use of iron oxide as an oxygen carrier in chemical combustion of methane with inherent separation of CO2[J]. Fuel, 2001, 80 (13): 1953-1962
[8] Thomas F. Advance in Synthetic Organic Chemistry and Methods Reported in US Patents[M]. USA: Elsevier, 2006: 704
[9] Cormos C C. Evaluation of iron based chemical looping for hydrogen and electricity co-production by gasification process with carbon capturing and storage[J]. Internationl Journal of Hydrogen Energy, 2010, 35(6): 2278-2289
[10] Ryu H J, Jin G T. Chemical-looping hydrogen generation system: Performance estimation and process selection[J]. Korean J Chem Eng, 2007, 24(3): 527-531
[11] Cui Hongtao, Zayat M, Levy D. Epoxide assisted solgel synthesis of perovskite-type LaMxFe1-xO3(M=Ni, Co) nanoparticles[J]. Journal of Non-Crystalline Solids, 2006, 352:3035-3040
[12] Wang Huanxin, Xu Jiaqiang, Cheng Zhixuan, et al. Synthesis and H2S gas sensing properties of SmFeO3nanosized powder[J]. Chinese J Sensor Actuator, 2006, 19(5): 2138-2141(in Chinese)
Recieved date: 2012-12-27; Accepted date: 2013-02-05.
Liang Hao, Telephone: +86-24-56389767; E-mail: lianghao2003@126.com.
- 中国炼油与石油化工的其它文章
- Isolation and Characterization of a Thermophilic Oil-Degrading Bacterial Consortium
- Quantum Chemistry of PAHs Thermal Cracking with Different Hydrogenation Degree
- Experimental Study on Aqueous Phase Entrainment in a Mixer-settler with Double Stirring Mode
- Effects of Temperature Gradient and Cooling Rate on the Formation of Methane Hydrates in Coarse Sand
- Influence of the Alkali Treatment of HZSM-5 Zeolite on Catalytic Performance of PtSn-Based Catalyst for Propane Dehydrogenation
- Development and Commercial Application of Third Generation Resid Hydrotreating Catalysts

