Research on Acupuncture Regulation of Visual Cortex c-Fos Protein Expression in Monocular Deprivation Cats
Zhu Tian-tian, Ma Chong-bing, Liu An-guo, Dong Li-li, Wang Jun-yan, Yan Xing-ke
Gansu College of Traditional Chinese Medicine, Lanzhou 730000, China
Research on Acupuncture Regulation of Visual Cortex c-Fos Protein Expression in Monocular Deprivation Cats
Zhu Tian-tian, Ma Chong-bing, Liu An-guo, Dong Li-li, Wang Jun-yan, Yan Xing-ke
Gansu College of Traditional Chinese Medicine, Lanzhou 730000, China
Objective: To explore the mechanism underlying acupuncture regulation of the visual cortex plasticity.
Methods: Eighteen kittens of four weeks were randomly divided into 3 groups, a normal group, a model group and an acupuncture group, with six in each group. There was no treatment to those in the normal group. Unilateral eyelid suture method was used to establish the deprivation amblyopia cat model in the model group and the acupuncture group. After that, kittens in the model group didn’t receive any treatment, but those in the acupuncture group were treated with acupuncture therapy of 12 weeks. Pattern Visual Evoked Potential (P-VEP) and c-Fos protein expression of visual cortex of kittens in each group were tested before and after acupuncture treatment.
Results: P-VEP waveform changed significantly in kittens of the model group, the time value of P100was significantly delayed (P<0.01) and N45-P100amplitude was significantly lower (P<0.01) compared with the normal group. After treatment, the time value of P100in kittens of the acupuncture group was significantly shorter (P<0.01) and N45-P100amplitude was significantly higher (P<0.01) when compared with the model group. Expression of c-Fos positive neurons can be seen in the visual cortex layers II-IV of kittens in the acupuncture group, and the density and percentage of c-Fos immunoreactive neurons of cortex layers II-IV in kittens of the model group were significantly lower than those in the acupuncture group.
Conclusion: Acupuncture has obvious improvement for abnormal changes of P-VEP waveform of monocular visual deprivation kittens; it can also increase the c-Fos protein expression in visual cortex after formdeprived.
Acupuncture Therapy; Amblyopia; Proto-Oncogene Proteins C-Fos; Evoked Potentials, Vision
Amblyopia is a common eye disease in clinics that hinders children's visual development. The amblyopia incidence of abroad is reported as 2% to 4%, and about 40% to 60% of children with strabismus and anisometropia will develop into amblyopia if not treated timely, and with the incidence increasing year by year[1]. The amblyopia seriously affected the healthy development of children's visual function, hindered the improvement of the quality of the population, and it is one of the most serious ophthalmic problems demanding prompt solution.
Acupuncture is effective in the clinical treatment of amblyopia and can significantly improve pattern visual evoked potential (P-VEP) response amplitude and latency in children with amblyopia. It is a safe, simple, practical, short course and quick therapy, and able to make up for other therapy. In addition, it is easy to adapt for infants and young children with satisfactory outcome[2-3].
Therefore, the present experiments used deprivation amblyopia cat as animal model, employ visual electrophysiology techniques and immunohistochemistry to detect P-VEP and c-Fos protein expression changes of visual cortex in the cat model before and after acupuncture, in order to reveal the mechanism of acupuncture in preventing and treating amblyopia, as well as adjusting visual system plasticity. The acupoints were selected based on years of clinical experience.
1 Experiment Animals and Materials
1.1 Animal selection and grouping
Eighteen healthy kittens (4 weeks old, provided by the Experimental Animal Center of the Academy of Military Medical Sciences) were selected with no limit on gender. All kittens were randomly divided into a normal group, a model group and an acupuncture group, with six in each group.
1.2 Laboratory instruments
Four-channel somatosensory potentials evoked system (Sunjava Medical Industry Co., Ltd., Guangzhou, China), visual evoked potential detection software system (Institute of Brain Science, Fudan University, Shanghai, China), Humphrey automatic perimetry (Allergan, Inc., USA), brain stereotaxic apparatus (Stoelting, USA), copper mesh shield (Huaibei Zhenghua Instrument, Anhui Province, China), the HHS electric constant temperature water bath (The 5th Medical Apparatus Factory, Shanghai, China), freezing microtome (Leica, Germany), BX51 Olympus microscope (Olympus, Japan).
1.3 Experiment reagents
Chloral Hydrate (provided by Yangzhou Aoxin additives plant), Methylcellulose eye drops, Chloramphenicol eye drops, Erythromycin ointment, Saline, Tropicamide eye drops, Atropine injection, Phenylephrine eye drops, Penicillium injection and 50% Glucose Saline, etc.
2 Experiment Methods
2.1 Establishment of the model
An animal model of deprivation amblyopia was established according to the literature[4]. Kittens in the model group and acupuncture group were anesthetized by intraperitoneal injection of 3.5% chloral hydrate [300 mg/(kg·bw)]. Unilateral upper and lower eyelid margins of 1 mm tissue were cut off from the inner canthus to the outer canthus of left eye. The upper and lower eyelids wound were closed with intermittent mattress suture. Postoperative eyelids were checked twice a day. Kittens with wound infection or loosened suture causing light leakage were excluded from the experiments.
2.2 Grouping and treatments
2.2.1 Normal group
Kittens in the normal group were caged and fed separately in natural visual environment. P-VEP was detected and kittens were sacrificed after 12 weeks.
2.2.2 Model group
Monocular deprivation model was replicated in kittens of the model group in the fourth week (reference part 2.1). After that, kittens were caged and fed separately in nature visual environment. P-VEP was detected and kittens were sacrificed after 12 weeks.
2.2.3 Acupuncture group
Acupoints: Jingming (BL 1), Cuanzhu (BL 2), Fengchi (GB 20), Guangming (GB 37).
Operations: Acupoints were selected according to the Experimental Acupuncture Science[5], and bilateral acupoints were used alternatively. Treatment started from the 1st day after operation, with acupuncture needles of 0.30 mm in diameter and 25 mm in length. Jingming (BL 1), Fengchi (GB 20) and Guangming (GB 37) were punctured straight to 5-10 mm, and needles were obliquely inserted in Cuanzhu (BL 2) to 8-10 mm. Acupuncture treatment was applied daily and 10 times as a course. The treatment course interval was two days. The whole process lasted for 12 weeks.
3 Outcome Measures
3.1 P-VEP detection of kittens in all groups
3.1.1 P-VEP detection procedures
The kittens were weighed and given chloral hydrate by body weight (4%, intraperitoneal injection of 0.2 mL/100 g). Two drops of Tropicamide and Phenylephrine were given to induce mydriasis instantaneous eyelid film contraction. During detection, the animal was fixed and the binocular axis was adjusted to make the corneal light reflection point parallel to the screen center markers.
3.1.2 P-VEP testing standards
Pattern reversal stimulator settings: Stimulation frequency was 4 Hz; pattern mode was checkerboard; graphic size was 8 × 6 and full vision.
Amplifier settings: The upper limit frequency was 500 Hz; lower limit frequency was 1 Hz; recordingelectrode was OZ; reference electrode was Fpz; sampling schedule was 300 ms; and superimposed number was 128 times. The impedance between the electrode and the reference electrode was ≤5Ω.
Stainless steel needle electrodes were used. Recording electrodes were placed 1.5 cm above the occipital protuberance and the reference electrode was inserted into the middle of the forehead between the eyebrows. The distance between the test cornea and the vertical screen was 57 cm when electrodes were stimulated.
3.2 Detection of c-Fos protein expression of visual cortex
3.2.1 Visual cortex specimen obtaining and preparation
At the end of the experiment, the head of kittens under full anesthesia was placed on the ice box and decapitated quickly from the pillow atlantoaxial joints. Whole brain tissue was removed and repaired to 1/3 size, from optic chiasma to the front of the cerebellum. After trimming, the brain tissue was successively sinking through 10% sucrose, 20% sucrose and 30% sucrose. Six frozen sections with thickness of 10 μm were prepared. Two for HE staining and two for immunohistochemical staining. HE staining steps were according to a conventional method.
3.2.2 Immunohistochemistry
Avidin-Biotin-Peroxidase Complex (ABC) method was used for c-Fos protein immunohistochemical staining. Strept-Avidin-Biotin-Peroxidase Complex, SABC) immunohistochemistry kit was provided by Zhanchen Company, China. The primary anti-c-Fos monoclonal antibody (ABCAM Company, UK), the secondary anti-biotin-labeled goat anti-mouse IgG and the third anti-streptavidin horseradish peroxidase labeled avidin were used. The followed procedure was DAB chromogenic, staining, mounting and microscopic examination. The negative control was realized by replacing the primary antibody with Tris-HCl buffer and the positive slide was provided by the company. Integral absorbance and number density of c-Fos protein in layers II-VI of the visual cortex OclM and OclB were detected using the Leika Q-Win image analysis system with 40 × 10 times light microscope. Number density was represented with immunoreactive cells/mm2. Five fields were randomly selected on each slice.
3.3 Statistical analysis
The SPSS 11.0 statistical software was used for data analysis. Measurement data were expressed as mean ± standard deviation (), and analyzed data using One-way analysis of variance and Q test. P<0.05 was considered statistically significant.
4 Results
4.1 P-VEP test results of kittens in all groups
Compared with the normal group, the P100time value of P-VEP wave crest in the model group was significantly delayed and the difference was statistically significant (P<0.01), indicating that the monocular visual deprivation model was successfully replicated. After acupuncture treatment, the P100time value in the acupuncture group reduced compared with the model group, and the difference was statistically significant (P<0.01), while the difference was not statistically significant between the acupuncture group and normal group, indicating that acupuncture can antagonize the effects of visual deprivation (table 1).
The N45-P100amplitude waveform in the model group was significantly lower when compared with the normal group, and the difference was statistically significant (P<0.01), indicating the visual dysfunction present after monocular visual deprivation. The N45-P100amplitude waveform in the acupuncture group was significantly higher when compared with the model group, and the difference was statistically significant (P<0.01), while the difference was not statistically significant between the acupuncture group and normal group, indicating that during the visually sensitive period, acupuncture can antagonize the effect of visual deprivation (table 1).
Table 1. P-VEP comparison of kittens in all groups ()
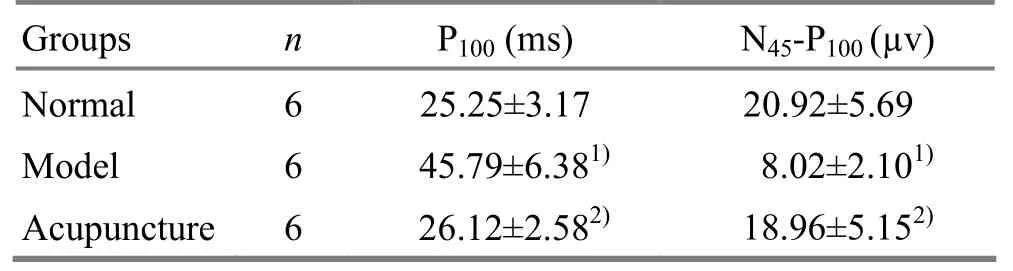
Table 1. P-VEP comparison of kittens in all groups ()
Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01
Groups n P100(ms) N45-P100(µv) Normal 6 25.25±3.17 20.92±5.69 Model 6 45.79±6.381)8.02±2.101)Acupuncture6 26.12±2.582)18.96±5.152)
4.2 C-Fos expression in the visual cortex of amblyopic cats
C-Fos expression of immunoreactive neurons was observed in layers II-IV of the visual cortex OclM and OclB region of the normal group kittens under light microscope: Nerve cells were arranged in neat rows in a relatively dense way. C-Fos immunoreactive neurons expression of visual cortex of kittens in the model group decreased, the cells arranged sparsely and cell gap increased. More immunoreactive neurons in layers II-IV of the visual cortex OclM and OclB regions of the acupuncture group can be seen. The nucleus of the neurons was dark brown and the cytoplasm, axons and dendrites were not colored or in lighter color (Fig.1).

Fig.1 C-Fos protein expressions were detected by immunohistochemistry (DAB staining, ×400)
C-Fos immunoreactive neurons density and percentage of positive cells in layers II-IV of the visual cortex of kittens in the model group were lower than those in the normal group or acupuncture group (P<0.05). Positive cell density and absorbance of c-Fos protein expression neurons in kittens visual cortex of the three groups showed statistically significant difference (P<0.05), (table 2).
Table 2. Comparison of c-Fos positive cell density and integral absorbance in visual cortex of kittens in all groups ()
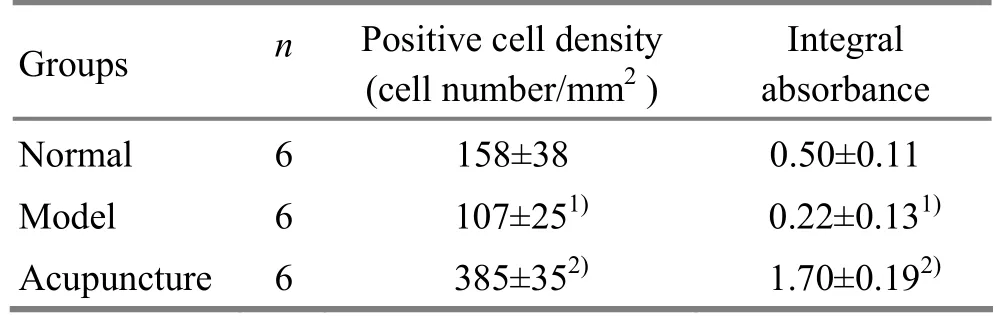
Table 2. Comparison of c-Fos positive cell density and integral absorbance in visual cortex of kittens in all groups ()
Note: Compared with the normal group, 1) P<0.01; compared with the model group, 2) P<0.01
Groups n Positive cell density (cell number/mm2) Integral absorbance Normal 6 158±38 0.50±0.11 Model 6 107±251)0.22±0.131)Acupuncture 6 385±352)1.70±0.192)
5 Discussion
The present experiment established monocular deprivation amblyopia cat model by unilateral eyelid suture in 4-week old kittens, and detected the P-VEP and immediate early gene c-Fos protein expression of the deprived eye before and after acupuncture treatment to reveal the mechanism of acupuncture prevention and treatment of amblyopia in view of visual system plasticity.
P-VEP is the electrical activity recorded when passing through the visual pathway to the occipital visual cortex in the cerebral cortex, induced when eyes fixed on the black and white reversal stimuli pattern. It reflects the functional status from the retinal ganglion cells to the visual cortex, and it is an important way to check the visual impairments. Generally, the P-VEP wave amplitude reflects the patient's visual acuity, and the latency reflects the optic nerve conduction. If the visual sense develops normally, the P1crest latency will gradually become shortened and the N1-P1amplitude will gradually become larger[6]. Whereas the N-wave and P-wave latency of P-VEP of the amblyopic eye extend significantly compared with the normal eyes, P100wave crest amplitude was significantly decreased compared with normal eyes[7]. The results of the present study show that 4 weeks after monocular visual deprivation model was established, P-VEP waveform of the model group significantly changed, manifested as that the time value of P100significantly delayed and N45-P100amplitude was significantly reduced compared with the normal group, indicating monocular visual deprivation model was successfully replicated. After acupuncture treatment, there were significant changes of the time value and the amplitude: the P-VEP time value of P100of the acupuncture group appears significantly earlier (P<0.01) compared with the model group, and N45-P100amplitude was significantly higher (P<0.05), indicating that during the sensitive period of visual development, acupuncture can antagonize the damage on visual system of the visual deprivation effect.
C-Fos is an immediate early gene at a low level of basal expression, but its expression level increases rapidly after stimulation. It can express rapidly and transiently when it is stimulated, therefore, c-Fos protein can be used as the excitement sign of visual cortex neurons, and c-Fos protein expression level can reflect the developmental plasticity degree of visual cortical neurons[8-9]. C-Fos protein immunohistochemical staining showed that in visual cortex slices of normal kittens, the nerve cells are arranged in a hierarchy of different shades of color, showing a typical sub-zonal arrangement. There were barely neurons in layer I, relatively more neurons in layers II and III, most intensive in layer IV and less in layer V. In view of this, the count and statistics were based on observation of layers II-VI except to layer I.
The end time of cat visual plasticity critical period is 16 weeks after birth. The present experiment used 4 weeks kittens to full replication of the amblyopia model for the experiment. The experimental results show that c-Fos expression of immunoreactive neurons was observed in II-IV layers of the visual cortex OclM and OclB regions of the normal group kitten under light microscope: nerve cells were arranged in neat rows in a relatively dense way. C-Fos immunoreactive neurons expression of visual cortex of the model group kittensdecreased and cells were arranged sparsely and cell gap increased. More immunoreactive neurons in layers II-IV of the visual cortex OclM and OclB regions of the acupuncture group can be seen. The nucleus of the neurons was dark brown and the cytoplasm, axons and dendrites were not colored or in lighter color.
The density and positive cells percentage of c-Fos immunoreactive neurons in visual cortex layers II-IV of kittens in the model group was significantly lower than those in the acupuncture group, indicating that after visual deprivation, the visual cortex neuronal excitability reduced and c-Fos protein expression decreased due to lack of light stimulation. Acupuncture stimulation may increase cortical neuronal excitability and thus effectively antagonize the deprivation effect during sensitive period of visual development, inhibit neuron degeneration in plastic period to reverse the damage caused by deprivation and to adjust the disorder of the visual environment, thus it can promote the development of neurons and synapses and establish a good visual development model[10-12]. It is suggested that acupuncture can effectively antagonize deprivation effect during the sensitive period of visual development. These results provide new ideas and methods for the mechanism study of acupuncture prevention and treatment on amblyopia, and will also have a positive role in promoting clinical application and mechanism studies on acupuncture prevention and treatment of amblyopia.

[1] Von Noorden GK. The development of the art and science of strabismology outside North America: part I. J AAPOS, 2001, 5(2): 65-69.
[2] Ge HL, Liu SQ. Observation of intractable amblyopia treated by acupuncture. Shijie Zhongxiyi Jiehe Zazhi, 2009, 4(8): 567-569.
[3] Yan XK, Chu HJ, Wang FC, Yang B, Gao Y. Point electric stimulation and children's amblyopia. J Acupunct Tuina Sci, 2007, 5(3): 147-151.
[4] Wang HF, Wang FC, Shi Y. Study on the correlation between the deprivation effect of resisting amblyopia of acupuncture and brain derived neurotrophic factor. Zhenci Yanjiu, 2005, 30(4): 208-211.
[5] Lin WZ, Wang P. Experimental Acupuncture Science. Shanghai: Shanghai Scientific and Technical Publishers, 1994: 287.
[6] Qiu FY, Xue Y, Wang LP. System of examination and treatment of amblyopic eyes based on P-VEP technology. Dianzi Celiang Yu Yiqi Xuebao, 2006, 20(1): 36-39.
[7] Wu XY, Luo YL, Liu DL, Liu SZ. Effects of levodopa on visual evoked potential and visual cortex neuron in monocular deprivation rat. Zhonghua Shiyan Yan Ke Zazhi, 2011, 29(3): 220-225.
[8] Tanaka S, Ribot J, Imamura K, Tani T. Orientationrestricted continuous visual exposure induces marked reorganization of orientation maps in early life. Neuroimage, 2006, 30(2): 462-477.
[9] Mower GD, Kaplan IV. Immediate early gene expression in the visual cortex of normal and dark reared cats: differences between Fos and Egr-1. Brain Res Mol Brain Res, 2002, 105(1-2): 157-160.
[10] Chen C, Cui HF, Yan XK, Wang FC. Advances in clinical research on acupuncture treatment of amblyopia. Shanghai Zhenjiu Zazhi, 2011, 30(1): 64-67.
[11] Wang HF, Wang FC, Shi Y. Deprivation effect of acupuncture against amblyopia and its mechanism. Zhongguo Linchuang Kangfu, 2004, 8(35): 8036-8037.
[12] Tao XY, Ru K, Lang S, Zhu WL, Fu J, Chen J, Ye C, Tao Y, Li Y, Yu YH. Observations on the efficacy of acupuncture at periocular extraordinary points in treating juvenile myopia. Shanghai Zhenjiu Zazhi, 2010, 29(10): 643-645.
Translator: Feng Xiao-ming
R2-03
A
Date: February 15, 2013
Author: Zhu Tian-tian, 2012 grade master degree candidate
Yan Xing-ke, M.D., professor.
E-mail: yanxingke@126.com
 Journal of Acupuncture and Tuina Science2013年3期
Journal of Acupuncture and Tuina Science2013年3期
- Journal of Acupuncture and Tuina Science的其它文章
- Clinical Observation on Treatment of Insomnia with Puncturing Back-Shu Acupoints
- Clinical Observation on Deep Acupuncture at Huantiao (GB 30) for Patients with Chronic Prostatitis
- Clinical Observation on Acupuncture for Perimenopausal Syndrome
- Therapeutic Efficacy Observation on Acupuncture for Postmenopausal Osteoporosis
- Research Progress of Acupuncture-moxibustion for Insomnia: An Analysis of Literature in Recent 5 Years
- Therapeutic Efficacy Observation on Integrative Acupuncture Therapy for Chronic Urticaria
