下调miR-21表达抑制鼻咽癌CNE2细胞增殖和侵袭
南华大学附属南华医院急诊科,湖南 衡阳421002
下调miR-21表达抑制鼻咽癌CNE2细胞增殖和侵袭
周克兵 谷刚 曹昕
南华大学附属南华医院急诊科,湖南 衡阳421002
背景与目的:miR-21在人类多种肿瘤中异常高表达。本研究探讨干扰miR-21表达对鼻咽癌CNE2细胞增殖、迁移和侵袭的影响。方法:使用脂质体将miR-21 inhibitor转染CNE2细胞,以无关序列(NC inhibitor)作为阴性对照。采用qRT-PCR技术验证miR-21 inhibitor转染的CNE2细胞中miR-21的表达水平;通过MTS法、细胞划痕、Transwell侵袭实验观察下调miR-21表达对CNE2细胞增殖、迁移和侵袭的影响。结果:转染miR-21 inhibitor的CNE2细胞中miR-21的表达明显下调,并且呈浓度依赖性。表明转染miR-21 inhibitor能有效抑制CNE2细胞中miR-21的表达。转染miR-21 inhibitor的CNE2细胞与对照细胞相比,增殖速度明显减慢,差异有统计学意义(P<0.05)。细胞划痕实验显示,下调miR-21表达抑制CNE2细胞迁移(P<0.05)。Transwell侵袭实验结果显示,下调miR-21表达抑制CNE2细胞侵袭(P<0.05)。结论:miR-21能促进鼻咽癌细胞增殖、迁移和侵袭,其可能在鼻咽癌的发生、发展中发挥重要作用。
鼻咽癌;miR-21;细胞增殖;细胞侵袭
miRNA是由长约19~24个核苷酸组成的非编码小分子RNA,它通过降解靶mRNA或抑制靶mRNA的翻译来调节靶基因表达。MiRNAs参与生命过程中一系列的重要进程,包括生长发育、细胞增殖、分化、细胞运动、新陈代谢等[1]。研究发现,人类多种肿瘤中存在miRNA基因异常或表达异常[2]。miRNA通过癌基因和抑癌基因参与细胞增殖、凋亡和分化的调控,在肿瘤的发生、发展过程中发挥着重要的生物学功能[3-5]。研究表明,miR-21在乳腺癌、肺癌、胃癌、胰腺癌等组织和细胞中呈高表达[6-9],其可能作为癌microRNA促进肿瘤的发生发展[10-11]。相对于对照鼻咽黏膜组织,miR-21在鼻咽癌组织中表达显著上调,并且与鼻咽癌临床进展及转移呈正相关[12]。本研究通过在鼻咽癌CNE2细胞中转染miR-21 inhibitor,探讨miR-21下调对鼻咽癌细胞增殖、侵袭、迁移的影响,从而初步揭示miR-21在鼻咽癌中的生物学功能。
1 材料和方法
1.1 主要材料
LipofectamineTM2000转染试剂购自美国Invitrogen公司。qRT-PCR miRNA 检测试剂盒购自上海吉玛生物公司。miRNA inhibitor为Ambion公司产品。miR-21 inhibitor序列为5’-UCAACAUCAGUCUGAUAAGCUA-3’,NC inhibitor序列为5’-CAGUACUUUUGUGUAGUA CAA-3’。RPMI-1640培养基购自Hyclone公司、胎牛血清购自杭州四季青公司。MTS细胞增殖和毒性检测试剂盒购自美国Promega公司。铺有基质胶的Transwell侵袭小室(8.0 μm孔径)购自BD Biosciences公司。
1.2 细胞培养
鼻咽癌细胞株CNE2为典型低分化鳞癌细胞,由本实验室保存,培养于含10%小牛血清的RPMI-1640,在95%湿度、CO2体积分数为5%、37 ℃条件下培养。
1.3 细胞转染
转染前1d将细胞接种于6孔板中,待贴壁细胞融合度达40%~60%时,根据LipofectamineTM2000说明书用无血清培养基进行转染。转染后6 h换新鲜完全培养基,继续扩大培养以用于后续实验。
1.4 qRT-PCR检测
逆转录按上海吉玛生物公司转录miRNA逆转录试剂盒进行,反应体系:总RNA 70 ℃预变性10 min,10×Buffer 2 μL,10 mmol/L MgCl24 μL,dNTP 2 μL,Ribonuclease Inhibitor 0.5 μL,AMV逆转录酶(20 U)0.6 μL,Oligo(dT)15 Primer 1 μL,总RNA 1 μg,无酶水至20 μL。反应条件:42 ℃ 1 h,95 ℃ 5 min,4 ℃ 10 min。
PCR反应体系(SYBR Premix Ex TaqTM 20 μL体系):SYBR Premix Ex TaqTM 10 μL,特异性引物(10 μmol/L) 0.4 μL,连通Primer (10 μmol/L) 0.4 μL,cDNA模板2 μL,蒸馏水加至20 μL。采用Bio-Rad IQ5实时定量PCR仪进行PCR反应,反应条件:95 ℃ 30 s;95 ℃延伸5 s,共40个循环,60 ℃退火30 s。miR-21特异性引物序列为5’-TAGCTTATCAGACTGATGTT-3’。
1.5 MTS法检测细胞增殖活性
MTS是一种四唑盐化合物,是一种常用的细胞存活和生长的检测方法[13]。其检测原理是活细胞线粒体中琥珀酸脱氢酶能够代谢还原MTS,生成紫色可溶于细胞培养液的甲瓒,可通过酶标仪测定甲瓒的吸光度值来确定活细胞的相对数量。
miR-21 inhibitor转染CNE2细胞24 h后,将细胞接种于96孔板中,每组设6个复孔,放置37 ℃、CO2体积分数为5%的培养箱中培养。在未接种细胞的孔中加入RPMI-1640培养基中作为调零孔。接种后24、48、72和96 h各检测1次。检测时每孔加20 μL MTS检测试剂,37 ℃温育2 h,用酶标仪测定570 nm波长吸光度值(A570),并绘制生长曲线观察miR-21下调对鼻咽癌细胞CNE2增殖的影响。实验重复3次。
1.6 细胞划痕实验
miR-21 inhibitor转染CNE2细胞24 h后,将细胞接种24孔板中,待细胞融合度达到90%时,用10 μL移液枪枪头,以载玻片作依靠在每孔中心轴进行划痕并拍照(0 h),37 ℃、CO2体积分数为5%培养箱中继续培养24 h并拍照,测量划痕愈合程度。实验重复3次。
1.7 Transwell侵袭实验
铺有基质胶的Transwell侵袭实验可用于观察细胞侵袭性[14]。miR-21 inhibitor转染CNE2细胞24 h后,消化细胞,用无血清培养基洗涤2次,再用无血清培养基重悬细胞,细胞计数,调整细胞数为1×105/mL。在Transwell下室加入800 μL含15%FCS的培养基。在Transwell上室加入250 μL细胞悬液,37 ℃培养48 h。取出Transwell,擦掉靠上室侧膜的细胞,PBS洗涤,将Transwell用4%甲醛溶液固定10 min,结晶紫染色,显微镜下观察、照相,随机选取5个高倍视野(×200)进行细胞计数,并计算平均值。实验重复3次。
1.8 统计学处理
采用统计软件SPSS 13.0进行统计学分析。结果数据以x±s 表示,两组间比较采用t检验;3组或3组以上比较采用One-way ANOVA检验。P<0.05为差异有统计学意义。
2 结 果
2.1 转染miR-21 inhibitor有效抑制CNE2细胞中miR-21表达
首先,运用qRT-PCR方法分析鼻咽癌CNE2细胞与正常鼻咽上皮细胞NP69细胞中miR-21表达情况,结果发现CNE2细胞中miR-21表达明显高于NP69细胞,约为NP69的5.3倍(图1)。然后分别用NC inhibitor及miR-21 inhibitor(终浓度为10、20、40 μmol/L)转染CNE2细胞,48 h后抽提细胞总RNA,运用qRTPCR验证miR-21 inhibitor转染的细胞中miR-21的表达。结果表明,转染不同浓度miR-21 inhibitor的CNE2细胞中,miR-21的表达明显下调,并且呈浓度依赖性(图2)。表明miR-21 inhibitor能有效抑制CNE2细胞中miR-21的表达。
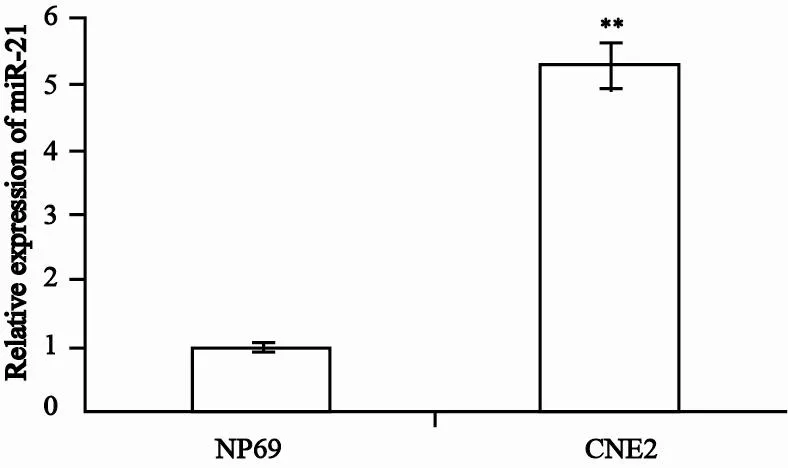
图 1 qRT-PCR检测CNE2和NP69细胞中miR-21表达水平Fig. 1 Analysis of miR-21 expression in CNE2 and NP69 cells by qRT-PCR

图 2 qRT-PCR验证miR-21 inhibitor转染细胞后miR-21表达水平Fig. 2 Analysis of miR-21 expression in CNE2 cells transfected with miR-21 inhibitor by qRT-PCR
2.2 miR-21下调后CNE2细胞增殖速度减慢
MTS法检测结果发现,转染不同浓度miR-21 inhibitor的CNE2细胞从第48小时起增殖速度明显减慢。统计分析各组在96 h时的细胞增殖情况发现,转染10、20、40 μmol/L的miR-21 inhibitor组A570分别为1.18±0.09、0.98±0.09、0.71±0.06,与对照细胞组(1.51±0.12)相比,差异均有统计学意义(P<0.05),并且呈现明显的浓度依赖性(图3)。这表明下调miR-21表达抑制鼻咽癌细胞CNE2增殖。
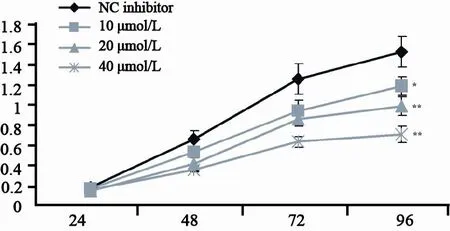
图 3 下调miR-21对CNE2细胞增殖的影响Fig. 3 Effect of miR-21 downregulation in CNE2 cells on cell proliferation
2.3 miR-21表达下调抑制CNE2细胞迁移
细胞划痕实验显示划痕后24 h,转染miR-21 inhibitor(20 μmol/L)的CNE2细胞划痕愈合率明显低于对照组细胞(P<0.05,图4),表明下调miR-21表达明显抑制CNE2细胞的迁移。
2.4 下调miR-21表达抑制CNE2细胞侵袭
本研究利用铺有基质胶的Transwell小室观察miR-21下调对细胞侵袭的影响。结果显示,转染miR-21 inhibitor(20 μmol/L)的CNE2细胞较对照细胞侵袭力明显降低,差异有统计学意义(P<0.05,图5)。
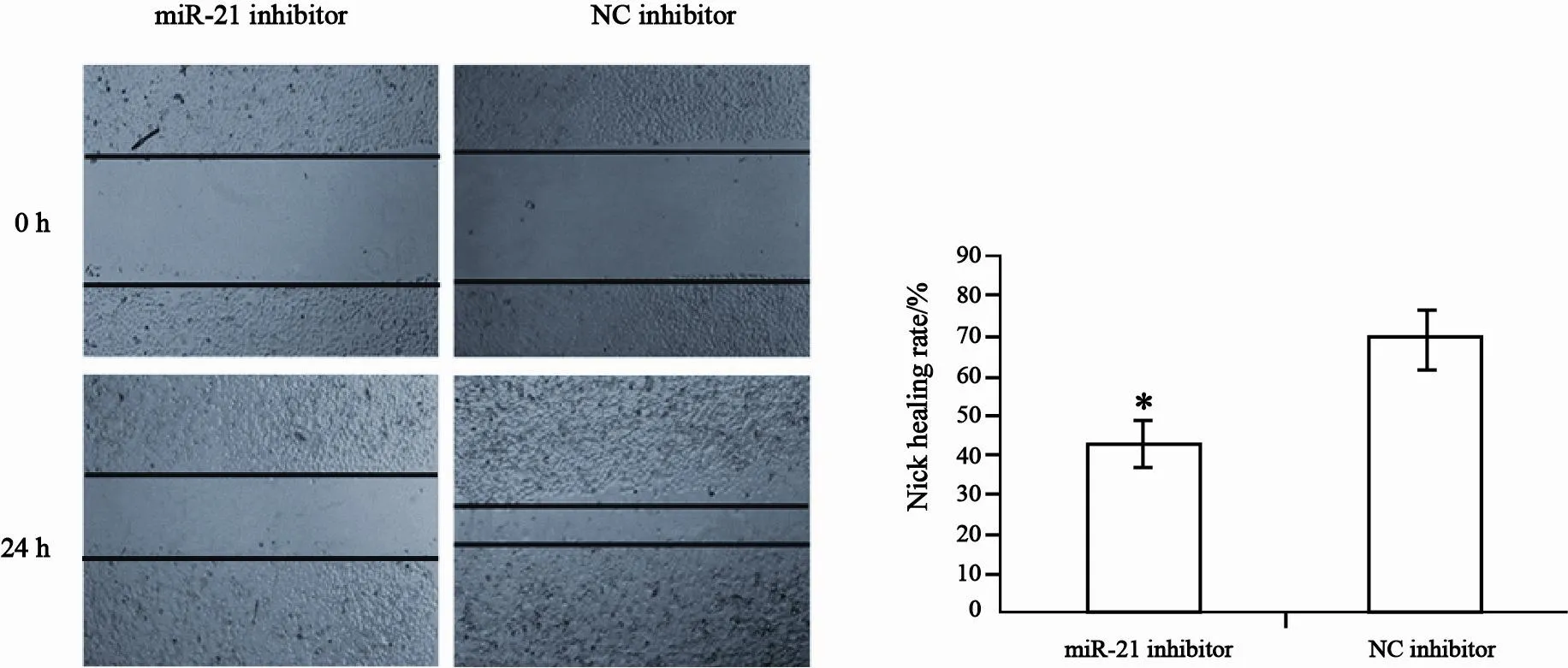
图 4 miR-21表达下调对CNE2细胞迁移的影响Fig. 4 Effect of miR-21 downregulation in CNE2 cells on cell migration
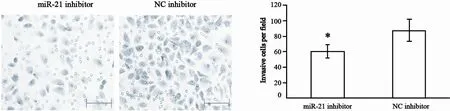
图 5 miR-21表达下调对CNE2细胞侵袭的影响Fig. 5 Effect of miR-21 downregulation in CNE2 cells on cell invasion
3 讨 论
miRNA是一类广泛存在于动植物体内的非编码小RNA,主要参与基因转录后水平调控,通过与其目标mRNA分子的3'端非编码区域(3'-untranslated region,3'UTR)互补匹配降解靶mRNA或抑制其翻译。miRNA作为一类新型基因调控剂,与肿瘤的发生、发展密切相关。越来越多的研究显示,在人类肿瘤中存在miRNA表达的失调,miRNA通过癌基因和抑癌基因参与细胞增殖、凋亡和分化的调控,在肿瘤的发生、发展过程中发挥着重要的生物学功能。
miR-21是最早发现的哺乳动物microRNAs之一,成熟miR-21在不同物种之间高度保守。研究表明,miR-21在肺癌、前列腺癌、结肠癌、胃癌、肝癌等多种恶性肿瘤中过表达。Toiyama[15]等报道,外周血miR-21可能是结直肠癌的诊断和预后标志物。邓敏等[12]报道,miR-21在鼻咽癌组织中异常高表达,其表达与鼻咽癌临床进展及转移呈正相关,Kaplan-Meier生存曲线分析发现,鼻咽癌患者miR-21表达越高,预后越差。这些结果提示,miR-21在鼻咽癌的发生、发展中起着重要作用。本研究将miR-21 inhibitor转染至鼻咽癌CNE2细胞中,观察miR-21下调对细胞增殖、迁移侵袭的影响。结果发现,转染不同浓度miR-21 inhibitor的CNE2细胞从第48小时起增殖速度明显减慢。细胞划痕及Transwell侵袭实验结果显示,miR-21表达下调抑制CNE2细胞迁移、侵袭性。这些结果表明,miR-21能促进鼻咽癌细胞增殖和侵袭,很可能作为癌基因参与鼻咽癌的发生、发展。文献报道miR-21作为癌miRNA能够通过调控靶基因如PTEN、CCL20、FAS,促进肺癌、胃癌、结肠、肾癌等肿瘤细胞增殖、生长、侵袭[7,9-11]。本研究结果与文献报道的一致,miR-21将有可能成为治疗鼻咽癌的潜在靶点。
[1] HE L, HANNON G J. MicroRNAs: small RNAs with a big role in gene regulation [J].Nat Rev Genet, 2004, 5(7): 522-531.
[2] CHEN P S, SU J L, HUNG M C. Dysregulation of MicroRNAs in cancer [J]. J Biomed Sci, 2012, 19(1): 90.
[3] MANIKANDAN J, AARTHI J J, KUMAR S D, et al. Oncomirs: the potential role of non-coding microRNAs in understanding cancer [J]. Bioinformation, 2008, 2(8): 330-334.
[4] CHIN L J, RATNER E, LENG S, et al. A SNP in a let-7 microRNA complementary site in the KRAS 3’ untranslated region increases non-small cell lung cancer risk [J]. Cancer Res, 2008, 68(20): 8535-8540.
[5] SENGUPTA S, DEN BOON J A, CHEN I H, et al. MicroRNA 29c is down-regulated in nasopharyngeal carcinomas, upregulating mRNAs encoding extracellular matrix proteins[J]. Proc Natl Acad Sci U S A, 2008, 105(15): 5874-5878.
[6] ZHU Q, WANG Z, HU Y, et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma [J]. Oncol Rep, 2012, 27(5): 1660-1668.
[7] ZHANG B G, LI J F, YU B Q, et al. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN [J]. Oncol Rep, 2012, 27(4): 1019-1026.
[8] DARIDO C, GEORGY S R, WILANOWSKI T, et al. Targeting of the tumor suppressor GRHL3 by a miR-21-dependent proto-oncogenic network results in PTEN loss and tumorigenesis [J]. Cancer Cell, 2011, 20(5): 635-648.
[9] VICINUS B, RUBIE C, FAUST S K, et al. miR-21 functionally interacts with the 3’UTR of chemokine CCL20 and downregulates CCL20 expression in miR-21 transfected colorectal cancer cells [J]. Cancer Lett, 2012, 316(1): 105-112.
[10] MEDINA P P, NOLDE M, SLACK F J. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma [J]. Nature, 2010, 467(7311): 86-90.
[11] HATLEY M E, PATRICK D M, GARCIA M R, et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21 [J]. Cancer Cell, 2010, 18(3): 282-293.
[12] 邓敏, 谷依学, 郑国沛, 等. 鼻咽癌组织中miR-21的表达变化及意义[J]. 山东医药, 2012, 52 (47): 10-12.
[13] GAROFALO M, ROMANO G, DI LEVA G, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers[J]. Nat Med, 2011, 18(1): 74-82
[14] DENG M, TANG H L, LU X H, et al. miR-26a suppresses tumor growth and metastasis by targeting FGF9 in gastric cancer [J]. PLoS One, 2013, 8(8): e72662.
[15] TOIYAMA Y, TAKAHASHI M, HUR K, et al. Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer[J]. J Natl Cancer Inst, 2013, 105(12): 849-859.
miR-21 downregulation attenuates cell proliferation, migration and invasion in nasopharyngeal carcinoma
ZHOU Ke-bing, GU Gang, CAO Xin (Emergency Department, Affiliated Nanhua Hospital, University of South China, Hengyang Hunan 421002, China)
ZHOU Ke-bing E-mail: 562136355@qq.com
Background and purpose: miR-21 is ovexpressed in various types of human cancers. This study was designed to investigate the effect of miR-21 knockdown on cell proliferation, migration and invasion of nasopharyngeal carcinoma (NPC) cell line CNE2. Methods: CNE2 was transfected with miR-21 inhibitor by LipofectamineTM2000. Meanwhile CNE2 was transfected with NC inhibitor as negative control. qRT-PCR was used to detect the miR-21 expression in these cells. The effects of miR-21 downregulation on cell proliferation, migration and invasion were evaluated by MTS, wound-healing Transwell and invasion assays. Results: miR-21 expression was remarkably downregulated in miR-21 inhibitor-transfected cells in concentration-dependent manner, indicating transfection with miR-21 inhibitor can effectively reduce expression level of miR-21 in CNE2 cells. Transfection of miR-21 inhibitor into CNE2 cells led to a significant decrease in cell proliferation rate compared with control cells (P<0.05). miR-21 downregulation results in reduction of cell migration(P<0.05). Moreover, the cell invasion by Transwell invasion assay was reduced in miR-21-downregulated cells relative to control cells. Conclusion: miR-21 can promote cell proliferation, migration and invasion of NPC cells. And it maybe plays an important role in tumorigenesis and development of NPC.
Nasopharyngeal carcinoma; MiR-21; Cell proliferation; Cell invasion
10.3969/j.issn.1007-3969.2013.11.002
R739.63
:A
:1007-3639(2013)11-0863-05
2013-06-11
2013-10-20)
国家自然科学基金(No:81101526)。
周克兵 E-mail: 562136355@qq.com

