Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
(马富)**(赵红建)
Department of Chemical Engineering, Ningxia Teachers University, Guyuan 756000, China
Supercritical Fluid Extraction of a Novel Template from Mesoporous Zirconia and the Effect on Porous Structure*
MA Fu(马富)**and ZHAO Hongjian(赵红建)
Department of Chemical Engineering, Ningxia Teachers University, Guyuan 756000, China
Mesoporous zirconia was synthesized by a new and simple method. Zirconium n-propoxide was used as the zirconium source. A small, inexpensive nonsurfactant, triethanolamine, was used as the template. The template was removed by thermal treatment in air and supercritical fluid extraction using CO2. The structure of the resulting materials was characterized by X-ray diffraction, transmission electron microscopy, and N2adsorption-desorption analyses. The materials are found to have narrowly distributed average pore diameters and wormhole-like pore channels. However, higher surface area and larger pore volume are exhibited after supercritical fluid extraction with CO2. The removal of the template by thermal treatment also leads to condensation and mild shrinkage of the zirconia framework.
mesoporous zirconia, triethanolamine, structure, calcination, supercritical fluid extraction
1 INTRODUCTION
In recent years, great efforts have been devoted to the synthesis of mesoporous zirconia because of its use as a heterogeneous catalyst, catalyst support, adsorbent, chemical sensor, structural ceramics, etc. [1-5]. Most zirconia materials are prepared by the surfactant template technique to form mesostructure [6-10]. Some synthesis approaches involve the concepts of electrostatics, hydrogen bonding, and covalent-bond interactions. However, surfactant removal without the collapse of zirconium oxide framework is challenging [11, 12]. The template surfactant molecules are often removed using thermal treatments, usually in air between 673 and 873 K [6]. Such a procedure can remove completely the surfactant species, but lead to significant framework shrinkage, serious damage to the ordered structure, facile sinterability, and concomitant loss of surface. The major thermodynamic driving force is the crystalline transformation to dense, low-surface area monoclinic and metastable tetragonal phases [11-13]. An alternative method for template removal is supercritical fluid extraction (SFE) with CO2. This technique is environmentally friendly, requires relatively low temperature, allows the removal of templates without decomposition, and permits surfactant recovery and reuse [14].
In the present work, mesoporous zirconia with wormlike channels were synthesized via the hydrothermal route [15, 16]. This novel and simple method involved the use of triethanolamine (TEA), an inexpensive nonsurfactant chemical, as the template. This template was removed from the as-prepared materials by two different techniques, namely, thermal treatment in air and SFE with CO2. SFE with CO2was found to have comparable extraction effectiveness with that of the conventional thermal treatment method.
2 EX PERIMENTAL
2.1 Preparation
9.2 ml zirconium n-propoxide, 2.4 ml TEA, and 13.5 ml water were mixed at room temperature in a molar ratio of 1∶0.6∶25 to obtain a homogeneous mixture. After heating at 100 °C for 24 h in air, a solidified gel was formed, then transferred into an autoclave and heated at 150 °C for 48 h. The template was removed by the following procedures: (1) calcination at 600 °C for 10 h at a ramp rate of 1 °C·min−1in air, (2) 1 g of as-made material was treated with 76 g of CO2in a supercritical extraction system (Jiangsu Huaan HA121-50-02) stirred at 500 r·min−1with in 20 h at 313 K and 15 MPa.
2.2 Characterization
The X-ray diffraction (XRD) of the samples was recorded using a Brucker-AXS D8 Advance X-ray diffractometer with Cu Kαradiation. The transmission electron microscopy (TEM) images were recorded on a JEOL JME-2010. The nitrogen adsorption-desorption isotherms were measured using a Micromeritics ASAP 2010 system. The pore size distributions were obtained from the N2desorption branch isotherm using the Barrett-Joyner-Halenda (BJH) model.
3 RESUL TS AND DISCUSSION
3.1 X RD analysis
The XRD patterns of the samples are shown in Fig. 1. All solids show diffraction patterns with only one strong reflection around 1.2° in 2θ. Similar singlepeak diffraction patterns have been previously observed in mesoporous TUD-1 [17], indicating that the prepared sample has a mesoporous structure. These structures lack the regular channel packing [18]. However, there could also be a regular arrangement of wormholes within the particles. Assemblies of mesoporous molecular sieves containing wormhole structures with uniform channel diameters have been prepared over a range comparable to M41S materials [19]. In the low-angle scale at 3° to 7° 2θ in trace 1, peak broadening is clear, which may due to the relatively wide range of pore diameters and small particle sizes of the as-made materials after SFE with CO2. However, there is no similar phenomenon after calcination at 600 °C. This behavior is believed to be a result of the crystallization and growth of zirconia nanocrystallites in the mesoporous framework during the calcination process.
The wide-angle XRD pattern of the prepared sample in Fig. 1 (b) (trace 1) shows broad and weak diffraction peaks, indicating the emergence of tetragonal nanocrystalline zirconia in the amorphous framework. On the other hand, after calcination at 600 °C, well-crystallized zirconia with a tetragonal phase can be obtained. This result is different from previously reported mesoporous zirconia with an amorphous framework that are often prone to structural collapse during crystallization [6, 10]. However, the Brunauer-Emmett-Teller (BET) surface areas decrease after calcinations because of the increased material density induced by the crystallization of the zirconia framework.

Figure 1 XRD patterns of the sample, trace 1, SFE with CO2, trace 2, calcined at 600 °C for 10 h

Figure 2 TEM image of the sample (a) SFE with CO2, (b) calcined at 600 °C for 10 h
3.2 TEM analysis
The TEM images (Fig. 2) show worm-like or possibly sponge-like pore channels for the typical mesoporus ZrO2sample, which is in good agreement with the single diffraction peak in the XRD patterns. Similar pore channels have been observed for disordered mesoporous TiO2[20] and Ti-TUD-1 [21] also using TEA as the template. In general, the pores seemed to be packed together with no visible long-range order, as can be further confirmed by the TEM analysis. The CO2-SFE processed materials exhibited higher mesoporosities and substantially smaller pore sizes than the calcined samples, which maintain an integrated mesostructure even after thermal treatment at 600 °C for 10 h.
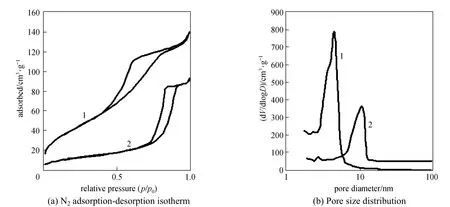
Figure 3 The typical N2adsorption-desorption isotherm and corresponding pore size distribution of mesoporous zirconia, trace 1, SFE with CO2, trace 2, calcined at 600 °C for 10 h
3.3 Nitrogen adsorption-desorption analysis
The typical N2adsorption-desorption isotherms and corresponding pore size distributions of the samples are shown in Fig. 3. The samples treated with CO2under supercritical conditions show obvious hysteresis loops and narrow pore size distributions similar to those exhibited by the calcined sample. The directly calcined sample has a BJH pore size of 8.7 nm, a BET surface area of 61 m2·g−1, and a total pore volume of 0.026 cm3·g−1. After SFE with CO2, the sample has a smaller BJH pore size of 4.3 nm. Particularly, the CO2-SFE processed materials exhibit a larger surface area (157.6 m2·g−1) and pore volume (0.223 cm3·g−1) than those directly calcined. The removal of the organic template using supercritical CO2leads to lower structural shrinkage than thermal calcination. Calcination may have caused further crystallization and the deformation of the crystalline framework, similar to the observations in Refs. [22, 23].
REFERENCES
1 Kuang, D.B., Brezesinski, T., Smarsly, B., “Hierarchical porous silica materials with a trimoidal pore system using surfactant templates”, J. Am. Chem. Soc.,126, 10534-10535 (2004).
2 Ikkala, O., Brinke, G., “Hierarchical self-assembly in polymeric complexes: Towards functional”, Chem. Commun.,16, 2131-2137 (2004).
3 Shin, Y., Liu, J., Wang, L.Q., Nie, Z., Samuels, W.D., Fryxell, G.E., Exarhos, G.J., “Ordered hierarchical porous materials: Towards tunable size- and shape-selective microcavities in nanoporous channels”, Angew. Chem. Int. Ed.,39(15), 2702-2707 (2000).
4 Schuth, F., “Non-siliceous mesostructured and mesoporous materials”, Chem. Mater.,13, 3184-3195 (2001).
5 Mamak, M., Coombs, N., Ozin, G., “Self-assembling solid oxide fuel cell materials: Mesoporous yttria-zirconia and metal-yttria-zirconia solid solutions”, J. Am. Chem. Soc.,122(37), 8923-8939 (2000).
6 Chen, H.R., Gu, J.L., Shi, J.L., Liu, Z.L., Gao, J.H., Ruan, M.L., Yan, D.S., “A composite surfactant route for the synthesis of thermally stable and hierarchically porous zirconia with a nanocrystallized framework”, Adv. Mater.,17, 2010-2014 (2005).
7 Hao, Z.P., Zhu, H.Y., Lu, G.Q., “Zr-laponite pillared clay-based catalysts for methane reforming with carbon dioxide”, Appl. Catal. A Gen.,242(2), 275-286 (2003).
8 Li, D.L., Zhou, H.S., Honma, I., “Design and synthesis of self-ordered mesoporous nanocomposite through controlled in-situ crystallization”, Nature Materials,3, 65-71 (2004).
9 Tian, B.Z., Liu, X.Y., Tu, B., “Self-adjusted synthesis of ordered stable mesoporous minerals by acid-base pairs”, Nature Materials,2, 159-163 (2003).
10 Yang, P., Zhao, D., Margolese, D.I., “Block copolymer templating syntheses of mesoporous metal oxides with large ordering lengths and semicrystalline framework”, Chem. Mater.,11(10), 2813-2826 (1999).
11 Schüth, F., “Non-siliceous mesostructured and mesoporous materials”, Chem. Mater.,13(10), 3184-3195 (2001).
12 Wong, M.S., Ying, J.Y., “Amphiphilic templating of mesostructured zirconium oxide”, Chem. Mater.,10(8), 2067-2077 (1998).
13 Yang, P.D., Zhao, D.Y., Margolese, D.I., Chmelka, B.F., Stucky, G.D.,“Generalized syntheses of large-pore mesoporous metal oxides with semicrystalline frameworks”, Nature,396(6707), 152-155 (1998).
14 Grieken, R., Calleja, G., Stucky, G.D., Melero, J.A., Garcia, R.A., Iglesias, J., “Fuid extraction of a nonionic surfactant template from SBA-15 materials and consequences on the porous structure”, Langmuir,19(432), 3966-3973 (2003).
15 Ma, F., Sun, J.H., Zhao, H.J., Li, Y., Luo, S.J., “Hydrothermal synthesis and characterization of mesoporous zirconia templated by triethanolamine”, Stud. Surf. Sci. Catal.,165, 301-304 (2007).
16 Sun, J.H., Ma, F., Zhao, H.J., Li, Y., Luo, S.J., “A new method synthesis mesoporous ZrO2”, CN Pat., 200610057129.1 (2006).
17 Jansen, J.C., Shan, Z., Marchese, L., Zhou, W., vanderPuil, N., Maschmeyer, T., “A new templating method for three-dimensional mesopore networks”, Chem. Commun.,37(8), 713-714 (2001).
18 Trens, P., Hudson, M.J., “Renaud denoyel formation of mesoporous, zirconium (IV) oxides of controlled surface areas”, J. Mater. Chem.,8(9), 2147-2152 (1998).
19 Prouzet, E., Pinnavaia, T.J., “Assembly of mesoporous molecular sieves containing wormhole motifs”, Angew Chem. Int. Ed.,36, 516-518 (1997).
20 Ma, C.F., Sun, J.H., Wang, F., “A new method synthesis mesoporous TiO2”, CN Pat., 2005100708798 (2005).
21 Shan, Z., Jansen, J.C., Marchese, L., Maschmeyer, T.H., “Synthesis, characterization and catalytic testing of a 3-D mesoporous titanosilica, Ti-TUD-1”, Micropor. Mesopor. Mater.,48(30), 181-187 (2001).
22 Chen, H.R., Shi, J.L., Zhang, W.H., Yuan, M.L., Yan, D.S., “Incorporation of titanium into the inorganic wall of ordered porous zirconium oxide via direct synthesis”, Chem. Mater.,13(3), 1035-1040 (2011).
23 Signoretto, M., Breda, A., Somma, F., Pinna, F., Cruciani, G., “Mesoporous sulphated zirconia by liquid-crystal templating method”, Micropor. Mesopor. Mater., 91, 23-32 (2006).
2011-08-09, accepted 2012-09-16.
* Supported by the Natural Science Foundation of Ningxia Province and Innovation Team Projects in Ningxia Teachers University.
** To whom correspondence should be addressed. E-mail: mfzhj@163.com
 Chinese Journal of Chemical Engineering2013年6期
Chinese Journal of Chemical Engineering2013年6期
- Chinese Journal of Chemical Engineering的其它文章
- Adsorption and Desorption Behavior of Tannic Acid in Aqueous Solution on Polyaniline Adsorbent*
- Synthesis of 2-Methyl-4-methoxyaniline from o-Nitrotoluene Using Pt/C and Acidic Ionic Liquid as Catalyst System*
- Preparation of Mesoporous Carbons from Acrylonitrile-methyl Methacrylate Copolymer/Silica Nanocomposites Synthesized by in-situ Emulsion Polymerization*
- Effect of Hydrogen Reduction of Silver Ions on the Performance and Structure of New Solid Polymer Electrolyte PEI/Pebax2533/AgBF4Composite Membranes*
- Comparison on Thermal Conductivity and Permeability of Granular and Consolidated Activated Carbon for Refrigeration*
- Immobilization of Papain in Biosilica Matrix and Its Catalytic Property*
