Application of acoustic radiation force impulse imaging for the evaluation of focal liver lesion elasticity
Changsha, China
Application of acoustic radiation force impulse imaging for the evaluation of focal liver lesion elasticity
Ping Zhang, Ping Zhou, Shuang-Ming Tian, Ying Qian, Jin Deng and Lu Zhang
Changsha, China
BACKGROUND:Acoustic radiation force impulse (ARFI) imaging is a new elastography method for the evaluation of tissue stiffness. This study aims to evaluate the performance of ARFI in noninvasive assessment of the tissue stiffness of focal liver lesion (FLL) and to explore its potential value in the differential diagnosis of FLL.
METHODS:ARFI was performed in 140 patients with 154 FLLs, which included 28 hemangiomas (ANGIs), 14 focal nodular hyperplasias (FNHs), 61 hepatocellular carcinomas (HCCs), 39 metastases and 12 cholangiocellular carcinomas (CCCs). Virtual touch tissue quantif i cation (VTTQ) values were obtained, analyzed and compared. The area under the receiver operating characteristic curve (AUROC) and optimal cut-off values were obtained using a receiver operating characteristic (ROC) curve analysis to assess diagnostic performance. All cases were def i nitively diagnosed using histopathology, CT, MRI or contrast-enhanced ultrasound.
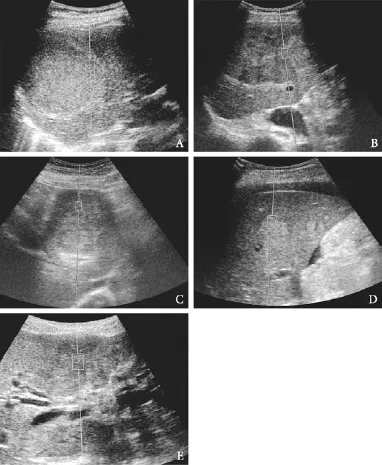
RESULTS:The VTTQ median values of ANGI, FNH, HCC, metastasis and CCC were 1.30, 1.80, 2.52, 3.08 and 3.89 m/s, respectively. A signif i cant increase in the VTTQ values of different lesions was observed: ANGI CONCLUSIONS:ARFI can accurately and objectively assess the elasticity of lesions by obtaining the shear wave elastic value of FLL with VTTQ. Therefore, ARFI is a novel, simple, noninvasive and useful diagnostic method for the characterization of FLL. (Hepatobiliary Pancreat Dis Int 2013;12:165-170) acoustic radiation force impulse imaging; elastosonography; focal liver lesion Elastography is a new ultrasound-based imaging modality that has aroused the interest of researchers in ultrasound imaging technology during the last two decades.[1-3]The theory of elastography originated from clinical palpation; the detection of tissue stiffness (i.e., elasticity) revealed the organization structure and vividly identif i ed the lesion location and the differentiation of lesion properties. Elastography is widely used for the differential diagnosis of superf i cial tissues, such as the breast, thyroid gland and prostate,[4-8]and the damage assessment of high-intensity focused ultrasound and radiofrequency ablation.[9-11] Various approaches to ultrasound elastography have been proposed over the years, including compression elastography (CE), transient elastography (TE), and acoustic radiation force impulse (ARFI) imaging.[12]The clinical feasibility of CE was fi rst demonstratedin vivoin 1991, but this technique was only applied to superf i cial organs to semiquantitatively detect the elasticity of tissues.[7,13]In 1999, Catheline et al[14]fi rst introduced TE to measure the elasticity of liver tissue. However, TE is inappropriate for the assessment of the tissue stiffness of FLLs because it is the one-dimensional elastography modality that cannot demonstrate the corresponding elastographic image and reveal the precise sample location. TE is restricted to the assessment of liverif brosis stage.[15,16] The virtual touch tissue quanti fi cation (VTTQ) of ARFI imaging is a new ultrasound elastographic imaging method. It involves the mechanical excitation of tissue using short-duration acoustic pulse (pushing pulse) in the region of interest (ROI) by the examiner, producing shear waves that spread away from the ROI. The VTTQ value of lesions is obtained by the tracking of shear wave velocity (SWV) through a series of diagnostic intensity pulses. The VTTQ value is proportional to the stiffness of the tissue because the SWV depends on tissue stiffness. VTTQ can not only quantify super fi cial but also deep tissue stiffness.[17-19]At present, the application of ARFI technology for the tissue stiffness of focal liver lesions (FLLs) has produced variable results.[20-24] The present study aims to evaluate the potential bene fi t of ARFI imaging for the characterization of FLLs. Patients Patients who had undergone ARFI elastography at the Third Xiangya Hospital of Central South University from March 2009 to July 2011 were enrolled in this study. A total of 160 patients with FLLs met the inclusion criterion: the patients had solid or mixed FLLs with a mean diameter greater than 1 cm. The following exclusion criteria were used: 1. the lesions were located at a depth greater than 8.0 cm from skin; 2. the lesions were near the heart and large blood vessels (the aorta or inferior vena cava); and 3. the patients had poor breathholds. Twenty FLLs were excluded. Therefore, a total of 140 patients (51 women and 89 men) with 154 FLLs with a lesion diameter of 1.1-11.1 cm (mean 4.0± 2.6) were included in this study. Their age ranged from 19 to 79 years (mean 51.6±12.3). Of the 140 patients, 132 patients had one lesion, 6 patients two lesions, and 2 patients three lesions. All patients were def i nitively diagnosed using histopathology, CT, MRI or contrastenhanced ultrasound. Biopsies were performed using an 18-gauge automated biopsy device with a 2.2-cm Tru-cut needle (MagnumTM; C.R. Bard, Inc., Covington, GA), and 2 or 3 cores were obtained from each nodule. Equipment and methods Ultrasound examination and ARFI imaging were performed using an Acuson S2000 ultrasound system (Siemens, Mountain View, CA., USA) with a 4.0 MHz convex array probe (4C1). All patients were required to fast before examination. Demographic data (age, gender) were obtained, and the location, size, depth, border and echo of the lesion were recorded using routine gray-scale sonography in all patients. The VTTQ of ARFI imaging was performed to quantify lesion stiffness. A greater VTTQ value (SWV) indicates an increase in tissue stiffness. The maximum VTTQ penetration depth was 8.0 cm, and the fi xed size of ROI was 10×6 mm. A ROI in the peripheral region of FLLs was chosen because the peripheral region is generally the stiffest region in the lesion. The patients were asked to hold their breath to minimize the breath motion when the VTTQ was performed. The VTTQ value was expressed in m/s, and the median value was obtained from 5 measurements per region. Two experienced sonographers with more than 5 years of experience in liver ultrasound performed all of the measurements. The sonographers were blind to the clinical and histological data. Ten patients with FLLs underwent two sets of VTTQ measurements to evaluate the reproducibility of VTTQ. The same sonographer performed the second evaluation at the same position of the FLL on the next day after the fi rst evaluation. Statistical analysis Statistical analysis was made using SPSS software (version 15.0, SPSS, Inc., Chicago, IL., USA). Data were expressed as mean±SD or the median (minimummaximum). The correlations between the VTTQ values of the lesions and both the diameter of the lesion and patient age were analyzed using the Spearman's rankorder correlation coeff i cient. The VTTQ values for each group of FLLs were compared using the Kruskal-Wallis test and ANOVA. The VTTQ value in diagnosis was determined using the sensitivity, specif i city and the receiver operator characteristic (ROC) curve. The ROC curve analysis identif i ed the cut-off value of differentiation for FLLs. The optimal cut-off value was chosen to maximize the sum of the sensitivity and specif i city on the Youden index. The area under the ROC curve (AUROC) and 95% conf i dence intervals were calculated using the Mann-WhitneyUtest. APvalue less than 0.05 was considered statistically signif i cant. Characteristics of patients No correlations were observed between the VTTQ values of lesions and patient age or lesion diameter (Table 1). Forty-two benign and 112 malignant lesions were identif i ed. The benign lesions included 28 hemangiomas (ANGI) and 14 focal nodular hyperplasias (FNHs). The malignant lesions included 61 hepatocellular carcinomas (HCCs), 39 metastases and 12 cholangiocellular carcinomas(CCCs). All the patients were conf i rmed by pathological examination or clinical imaging (Table 2). Clinical examination and imaging included a history of viral hepatitis, AFP, contrast-enhanced CT, MR and contrastenhanced ultrasound. Patients with HCC had a history of chronic hepatic disease, while other patients had no history of chronic hepatic disease. The 39 metastases originated from colon cancer (10 metastases), rectal cancer (6), lung cancer (13), pancreatic cancer (3), breast cancer (2), ovarian cancer (1), gallbladder cancer (1), and unknown origins (3). Reproducibility of VTTQ measurements The VTTQ values of 10 patients who had undergone two trials to evaluate the reproducibility of VTTQmeasurements are shown in Table 3. No signif i cant differences were observed between the mean VTTQ values in the fi rst and second trials. The coeff i cient of variation values was 1.8%-8.0%. Table 1.Correlation between VTTQ value of the lesions and patient age as well as lesion diameter Table 2.Pathological and clinical imaging results of FLLs VTTQ measurements The median VTTQ values were as follows (Fig. 1): 1.30 m/s (mean 1.33±0.38, range 0.74-2.21) for ANGI (Fig. 2A); 1.80 m/s (mean 1.90±0.45, range 1.22-2.80) for FNH (Fig. 2B); 2.52 m/s (mean 2.59±0.91, range 1.17-4.63) for HCC (Fig. 2C); 3.08 m/s (mean 3.20±0.62, range 2.22-4.36) for metastases (Fig. 2D); and 3.89 m/s (mean 3.74±0.54, range 2.76-4.36) for CCC (Fig. 2E). A signif i cant increase was observed in the VTTQ values of different lesions: ANGI Fig. 1.Box plots of VTTQ for different groups of FLLs. The top and bottom of the boxes are the fi rst and third quartiles, respectively. The length of the box represents the interquartile range, which includes 50% of the values. The line through the middle of each box represents the median. Hollow dots represent outliers. Table 3.Evaluation of reproducibility of VTTQ measurements Fig. 2.A: ANGI in a 42-year-old man. Baseline ultrasonography showing an inhomogeneous hyperechoic lesion in the sixth segment. The VTTQ value measured when the ROI was placed within the lesion was 1.30 m/s.B: FNH in a 38-year-old woman. Baseline ultrasonography showing an inhomogeneous hypoechoic lesion in the fifth segment. The VTTQ value measured when the ROI was placed within the lesion was 1.68 m/s.C: HCC in a 45-year-old man. Baseline ultrasonography showing an inhomogeneous hypoechoic lesion in the fifth segment. The VTTQ value measured when the ROI was placed within the lesion was 2.83 m/s.D: Metastasis in a 42-year-old woman. Baseline ultrasonography showing an inhomogeneous hyperechoic lesion in the fifth segment. The VTTQ value measured when the ROI was placed within the lesion was 3.63 m/s.E: CCC in a 48-yearold man. Baseline ultrasonography showing an inhomogeneous hypoechoic lesion in the fourth segment. The VTTQ value measured when the ROI was placed within the lesion was 3.77 m/s. Fig. 3.ROC curves of lesion stiffness determined by VTTQ for the diagnosis of ANGI (ANGI vs FNH+HCC+Metastasis+CCC), malignant lesion (ANGI+FNH vs HCC+Metastasis+CCC) and CCC (ANGI+FNH+ HCC+Metastasis vs CCC). ARFI imaging is an emerging modality for the visualization of the elastic properties (stiffness) of target tissues. Differences in tissue stiffness suggest various pathological processes, and the measurement of tissue stiffness might aid in the differentiation of various types of FLLs. Therefore, ARFI imaging may provide useful information for the study of liver lesions. Few papers have described the application of ARFI imaging for the identif i cation of the properties of FLLs.[20-24]It is reported that VTTQ values of liver lesions could be used to differentiate between benign and malignant liver lesions.[22]Cho et al[20]and Kapoor et al[24]also concluded that VTTQ values of liver nodules were useful in differentiating benign from malignant or metastatic nodules, and the VTTQ values depended on the tissue characteristics of the nodule rather than that of the surrounding liver parenchyma. Davies et al[21]reported that VTTQ values of patients with normal ultrasound examinations and normal liver enzymes were independent of site of measurement, age or gender, and were useful in differentiating ANGI from metastasis. However, these studies concentrated only on benign or malignant liver lesions or two types of elasticity-related lesions, and they failed to intensively evaluate the 5 different types of elasticity-related lesions against each other within the same study, as was done in the present study. The present study showed that the VTTQ values of metastases were greater than those of HCCs, similar to the previously reported VTTQ values of metastases and HCCs (3.28 and 2.40 m/s, respectively).[24]The mean VTTQ value of ANGI was 1.33±0.38 m/s, which is consistent with the VTTQ value of 1.35±0.48 m/s for ANGI that was reported previously.[21]However, our result was inconsistent with that reported by Cho et al,[20]i.e., a VTTQ value of HCC (2.45±0.81 m/s) that was greater than that of metastasis (2.18±0.96 m/s). This discrepancy may be due to sampling location. Because our samples were examined in the stiffest part of the peripheral region of lesions, rather than the central necrotic portion of metastasis. In the present study, the tissue stiffness of FLLs was CCC>metastasis>HCC>FNH>ANGI (P<0.001), illustrating that different tumors exhibited variable stiffness, malignant lesions were stiffer, and benign lesions were softer. This result is likely due to the constitutionof pathological tissues. Lesions that include fi brous contents are potentially stiffer, but lesions that include vessels tend to be softer. CCC was comparatively the stiffest lesion, since it is rich in fi brotic tissue instead of vessels. Metastasis is softer than CCC, depending on the amount of fi brotic tissue and vessels. HCC is composed of hepatocytes, rich vessels and less fi brotic tissue, and it is relatively the softest of malignant lesions. FNH is primarily composed of hyperplasic hepatic cells and rich vessels except for the central scar, and these lesions are relatively softer than malignant lesions. ANGI is the softest lesions primarily because it is composed entirely of vessels and sinusoids. The VTTQ values in this study were correlated with the pathological properties of tissues, and could accurately ref l ect the actual tissue stiffness. Moreover, the ROC curves revealed that the AUROCs of VTTQ for ANGI, liver malignant lesions, and CCCs were 0.94, 0.91, and 0.87, respectively. The AUROCs beyond 0.9 demonstrated higher diagnostic values. The AUROCs in our study were comparatively high, suggesting that the VTTQ values of FLLs were preferable for the differentiation between ANGI, liver malignant lesions, and CCCs. Therefore, AUROC was a good differential diagnosis index of FLLs. However, prospective studies with a larger population are required to conf i rm the diagnostic eff i ciency of the VTTQ value. Our study has some limitations. Some lesions were excluded from the study because of technical restrictions: 1. Several lesions were not detected since their locations were deeper than 8.0 cm from skin. Because high attenuation of the signal creates diff i culties for the system to consistently identify the SWV in deepseated lesions as it propagates.[22]2. Some lesions were not detected because of the interference of respiratory movement and impulses that arose from main blood vessels. 3. Several lesions were not detected due to obesity. The SWV signal has a higher attenuation in obese patients than in non-obese patients, thus resulting in poor quality images in obese patients.[26]4. The lesions with a mean diameter smaller than 1 cm were excluded because of the ROI fi xed at 10×6 mm. In conclusion, ARFI imaging can quantitatively assess the elasticity of lesions by obtaining the shear wave elastic value of FLLs with VTTQ. ARFI imaging provides useful information for the differential diagnosis of various FLLs, and the rapid development and improvement in ARFI technology will advance the clinical detection of FLLs. ARFI imaging is a promising diagnostic method for tumor detection and differentiation. Contributors:All authors contributed to the design and interpretation of the study and to further drafts. ZP is the guarantor. Funding:None. Ethical approval:Not needed. Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article. 1 Parker KJ, Doyley MM, Rubens DJ. Imaging the elastic properties of tissue: the 20 year perspective. Phys Med Biol 2011;56:R1-R29. 2 Das D, Gupta M, Kaur H, Kalucha A. Elastography: the next step. J Oral Sci 2011;53:137-141. 3 Oliver C, Vaillant-Lombard J, Albarel F, Berbis J, Veyrières JB, Sebag F, et al. What is the contribution of elastography to thyroid nodules evaluation? Ann Endocrinol (Paris) 2011;72: 120-124. 4 Cho N, Moon WK, Park JS, Cha JH, Jang M, Seong MH. Nonpalpable breast masses: evaluation by US elastography. Korean J Radiol 2008;9:111-118. 5 Fu LN, Wang Y, Wang Y, Huang YH. Value of ultrasound elastography in detecting small breast tumors. Chin Med J (Engl) 2011;124:2384-2386. 6 Lyshchik A, Higashi T, Asato R, Tanaka S, Ito J, Mai JJ, et al. Thyroid gland tumor diagnosis at US elastography. Radiology 2005;237:202-211. 7 Ding J, Cheng H, Ning C, Huang J, Zhang Y. Quantitative measurement for thyroid cancer characterization based on elastography. J Ultrasound Med 2011;30:1259-1266. 8 Brock M, von Bodman C, Sommerer F, Löppenberg B, Klein T, Deix T, et al. Comparison of real-time elastography with grey-scale ultrasonography for detection of organconf i ned prostate cancer and extra capsular extension: a prospective analysis using whole mount sections after radical prostatectomy. BJU Int 2011;108:E217-222. 9 Curiel L, Souchon R, Rouvière O, Gelet A, Chapelon JY. Elastography for the follow-up of high-intensity focused ultrasound prostate cancer treatment: initial comparison with MRI. Ultrasound Med Biol 2005;31:1461-1468. 10 Varghese T, Zagzebski JA, Lee FT Jr. Elastographic imaging of thermal lesions in the liverin vivofollowing radiofrequency ablation: preliminary results. Ultrasound Med Biol 2002;28: 1467-1473. 11 Kwon HJ, Kang MJ, Cho JH, Oh JY, Nam KJ, Han SY, et al. Acoustic radiation force impulse elastography for hepatocellular carcinoma-associated radiofrequency ablation. World J Gastroenterol 2011;17:1874-1878. 12 Li Y, Snedeker JG. Elastography: modality-specif i c approaches, clinical applications, and research horizons. Skeletal Radiol 2011;40:389-397. 13 Ophir J, Céspedes I, Ponnekanti H, Yazdi Y, Li X. Elastography: a quantitative method for imaging the elasticity of biological tissues. Ultrason Imaging 1991;13:111-134. 14 Catheline S, Thomas JL, Wu F, Fink MA. Diffraction fi eld of a low frequency vibrator in soft tissues using transient elastography. IEEE Trans Ultrason Ferroelectr Freq Control 1999;46:1013-1019. 15 Wong VW, Sung JJ. Application of transient elastography in chronic hepatitis B. Aliment Pharmacol Ther 2011;34:817-819. 16 Sporea I, Sirli R, Deleanu A, Popescu A, Cornianu M. Liverstiffness measurement by transient elastography in clinical practice. J Gastrointestin Liver Dis 2008;17:395-399. 17 Tozaki M, Isobe S, Fukuma E. Preliminary study of ultrasonographic tissue quantif i cation of the breast using the acoustic radiation force impulse (ARFI) technology. Eur J Radiol 2011;80:e182-187. 18 Friedrich-Rust M, Romenski O, Meyer G, Dauth N, Holzer K, Grünwald F, et al. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: a limited patient feasibility study. Ultrasonics 2012;52:69-74. 19 Berzigotti A, Ashkenazi E, Reverter E, Abraldes JG, Bosch J. Non-invasive diagnostic and prognostic evaluation of liver cirrhosis and portal hypertension. Dis Markers 2011;31:129-138. 20 Cho SH, Lee JY, Han JK, Choi BI. Acoustic radiation force impulse elastography for the evaluation of focal solid hepatic lesions: preliminary fi ndings. Ultrasound Med Biol 2010;36: 202-208. 21 Davies G, Koenen M. Acoustic radiation force impulse elastography in distinguishing hepatic haemangiomata from metastases: preliminary observations. Br J Radiol 2011;84: 939-943. 22 Shuang-Ming T, Ping Z, Ying Q, Li-Rong C, Ping Z, Rui-Zhen L. Usefulness of acoustic radiation force impulse imaging in the differential diagnosis of benign and malignant liver lesions. Acad Radiol 2011;18:810-815. 23 Fahey BJ, Nelson RC, Bradway DP, Hsu SJ, Dumont DM, Trahey GE.In vivovisualization of abdominal malignancies with acoustic radiation force elastography. Phys Med Biol 2008;53:279-293. 24 Kapoor A, Kapoor A, Mahajan G, Sidhu BS, Lakhanpal VP. Real-time elastography in differentiating metastatic from nonmetastatic liver nodules. Ultrasound Med Biol 2011;37: 207-213. 25 Palmeri ML, Nightingale KR. Acoustic radiation force-based elasticity imaging methods. Interface Focus 2011;1:553-564. Received April 11, 2012 Accepted after revision August 30, 2012 AuthorAff i liations:Department of Ultrasound, Third Xiangya Hospital, Central South University, Changsha 410013, China (Zhang P, Zhou P, Tian SM, Qian Y, Deng J and Zhang L) Ping Zhou, MD, PhD, Department of Ultrasound, Third Xiangya Hospital, Central South University, Changsha 410013, China (Tel: 86-731-88618403; Fax: 86-731-88618403; Email: zhouping1000@ hotmail.com) © 2013, Hepatobiliary Pancreat Dis Int. All rights reserved. 10.1016/S1499-3872(13)60027-2Introduction
Methods
Results





Discussion
 Hepatobiliary & Pancreatic Diseases International2013年2期
Hepatobiliary & Pancreatic Diseases International2013年2期
