Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects
Shanghai, China
Clinical operational tolerance in liver transplantation: state-of-the-art perspective and future prospects
Xi-Qiang Liu, Zhi-Qiu Hu, Yao-Fei Pei and Ran Tao
Shanghai, China
BACKGROUND:Liver transplantation is the def i nite treatment for end-stage liver diseases with satisfactory results. However, untoward effects of life-long immunosuppression prevent the development of alternative strategies to achieve better longterm outcome. Achieving clinical operational tolerance is the ultimate goal.
DATA SOURCES:A PubMed and Google Scholar search using terms: "immune tolerance", "liver transplantation", "clinical trial", "operational tolerance" and "immunosuppression withdrawal" was performed, and relevant articles published in English in the past decade were reviewed. Full-text publicationsrelevant to the fi eld were selected and relevant articles fromreference lists were also included. Priority was given to those articles which are relevant to the review.
RESULTS:Because of the inherent tolerogenic property, around 20%-30% of liver transplantation recipients develop spontaneous operational tolerance after immunosuppression withdrawal, and the percentage may be even higher in pediatric living donor liver transplantation recipients. Several natural killer and γδT cell related markers have been identif i ed to be associated with the tolerant state in liver transplantation patients. Despite the progress, clinical operational tolerance is still rare in liver transplantation. Reprogramming the recipient immune system by creating chimerism and regulatory cell therapies is among newer promising means to achieve clinical liver transplantation tolerance in the future.
CONCLUSION:Although clinical operational tolerance is still rare in liver transplantation recipients, ongoing basic researchand collaborative clinical trials may help to decipher the mystery of transplantation tolerance and extend the potential benef i ts of drug withdrawal to an increasing number of patients in a more predictable fashion.
(Hepatobiliary Pancreat Dis Int 2013;12:12-33)
immune tolerance; liver transplantation; clinical trial
Introduction
The past six decades have witnessed the exciting development of contemporary organ transplantation from dream to reality. The fi rst human liver transplantation (LT) was performed by Dr. Thomas Starzl in 1963, and it represented a true milestone in the history of modern medicine. After many years of hard work by the pioneers, especially with the development of better immunosuppression (IS), improvement of surgical techniques and perioperative care in the 1980s, LT has now been widely recognized as the most effective and ultimate management modality for acute and chronic irreversible liver disorders, with satisfactory short- and long-term survival rates. However, like many other organ transplantations, the overall life expectancy of LT recipients is still shorter than that of the general population. This can be largely attributed to the mandatory life-long IS to prevent graft loss. Indeed, current immunosuppressive drugs are responsible for the majority of long-term morbidity and mortality in transplant recipients. Their adverse-effects include nephrotoxicity, diabetes, cardiovascular diseases, metabolic syndrome, bone loss, etc, not to mention the signif i cant cost. In addition, these medications induce global, nonspecif i c IS, thereby predisposing the development of opportunistic infections or cancers. Finally, contemporary immunosuppressive therapy is still ineffective to prevent or treat chronic rejection,which remains a signif i cant cause of late graft loss. These untoward effects of life-long IS warrant the development of alternative strategies to achieve better long-term outcome in organ transplantation.
The ultimate answer to solve these problems is to induce donor-specif i c tolerance, def i ned as the longterm survival of an allograft without ongoing IS, albeit with preserved immune responses against other antigens. Immune tolerance has long been considered as the "holy grail" of transplantation medicine. Even if many methods of tolerance induction have succeeded in rodents, they are seldom successful in non-human primates or humans. Many different terms of tolerance have been described in the literature. "True tolerance" refers to the absence of any detectable detrimental immune response as well as the absence of immune compromise, which is the most rigorous def i nition derived from experimental transplantation studies. "Operational tolerance" or "functional tolerance" refers to the specif i c absence of a destructive immune response to a graft in the absence of IS, it is generally considered as the practical term for clinical tolerance. "Propetolerance" (almost tolerance), fi rst described by Calne and Watson after introduction of the powerful lymphocyte depletion agent alemtuzumab to clinical kidney transplantation, refers to a state of stable graft function in the presence of minimal dose of immunosuppressive agents. This very low dose of medication exposure may even be considered to be of no pharmacological signif i cance, but is critical for the well-being of the graft.[1]This term is not commonly used nowadays since operational tolerance has been found in more patients, also because of the instability of the "tolerance" state and the lack of knowledge of what exactly constitutes low level or minimal IS. They can generally be categorized as unsuccessful immunosuppressant withdrawal or nontolerant. Besides the many def i nitions of tolerance, there is no uniform consensus regarding how exactly the stability of function is or for how long stable function must be maintained for a recipient to be def i ned as tolerant. Transplantation tolerance has several typical features including prolonged allograft survival in the absence of all IS with normal graft function and histology, evidence of absent or suppressed donor-specif i c responses usingin vitroassays and the spontaneous acceptance of a second donor-derived graft but rejection of a third-party graft, etc.
Unlike in the case of acute or chronic rejection, our understanding of clinical operational tolerance is lacking. Factors affecting investigation of clinical transplantation tolerance include the lack of therapeutic regimens known to favor tolerance in humans, the lack of validated assays or biomarkers predictive of tolerance, and concerns regarding the safety and ethics of complete withdrawal of IS in otherwise very stable recipients. Withdrawal of IS following LT is not yet the standard of care, leaving most clinicians to maintain transplant patients on long-term IS therapy. However, sporadic tolerance can be observed in rare cases where there is non-compliance to immunosuppressive regimens or in which IS is deliberately withdrawn due to a research protocol or serious clinical concerns (e.g. lymphoproliferative disorder or life-threatening infections). This has lead to a growing interest in clinical tolerance studies in the past two decades. In recent years, the Immune Tolerance Network (ITN), an international consortium sponsored by the National Institutes of Health (NIH), and its European counterpart, the Reprogramming the Immune System for the Establishment of Tolerance (RISET) consortium created by the European Union, has devoted to advance both basic research and clinical trials of immune therapies for transplantation and autoimmune diseases through an innovative and collaborative effort. In the past decade, ITN and RISET have sponsored a series of clinical trials of immune tolerance based on valuable experience gained from early rodent studies.[2,3]
LT has unique advantages in immune tolerance studies partly due to the fact that the liver has long been considered as an immunologically privileged organ. Not only do many rodents accept fully MHC-mismatched liver allografts for an extended period without any immunosuppressive agents, but also liver transplants can protect other allografts transplanted simultaneously or sequentially. Moreover, the liver has a powerful regenerative capability upon injury, and acute rejections do not commonly have a negative impact on the longterm survival of the graft. Possible mechanisms of the tolergenic property of the liver allograft include the release of soluble MHC antigens, migratory passenger leukocytes and activation of recipient lymphocytes followed by apoptosis (activation induced cell death, AICD), microchimerism, hepatic dendritic cell immaturity, and stimulation of regulatory T cells.[4]Clinically, in comparison to other organs transplanted, liver allografts also have some immunological advantages. They are less likely to be rejected, hardly any hyperacute rejection has been reported. Liver allografts are more resistant to antibody-mediated rejection than other solid organ transplantations, therefore HLA-mismatched and certain ABO-incompatible transplants can be safely performed in most settings. A vast majority of acute cellular rejection episodes can be readily managed using current immunosuppressive medicationswithout signif i cant morbidity or mortality. Even the early phases of chronic rejection are reversible; reversal of rejection is usually complete, leaving no signif i cant fi brosis, architectural distortion or malfunction of the graft.[5]In the current review, we intend to brief l y summarize the major achievements and latest developments in clinical LT tolerance studies, including the discovery of spontaneous operational tolerance and the early results of IS weaning and withdrawal trials. We also review recent progress in tolerance monitoring and prediction. Finally, several promising strategies with potential clinical applicability will be discussed.
Clinical evidence of operational LT tolerance
One of the unique features in clinical LT is that spontaneous tolerance occurs more frequently than other types of grafts. Spontaneous operational tolerance refers to rare transplant recipients who are deliberately removed from IS due to medical, iatrogenic causes or noncompliance but do not develop rejection after a long-term follow-up. Since the original description of spontaneous operational tolerance by the Pittsburgh group in the early 1990s,[6,7]there have been sporadic reports of IS discontinuation after LT worldwide over the past two decades. Apparently, the actual number of spontaneous clinical LT tolerance should be larger than currently reported. Those patients either develop severe malignant (e.g. post-transplantation lymphoproliferative disorders), infectious or metabolic complications of chronic IS which mandate discontinuation of immunosuppressive medications or they are noncompliant with the prescribed drugs. The majority of these recipients who stop their anti-rejection medications develop acute or chronic allograft rejection which may eventually result in graft loss. However some patients have survived without need to resume the IS. In another rare circumstance, some patients received bone marrow transplantation (BMT) with achievement of stable chimerism, followed by LT months or years later without long-term IS (Table 1). Although these reports are not generalizable and hence have failed to revolutionize our patient management strategies, they do provide anecdotal evidence that a liver allograft may continue to function in the absence of IS. More importantly, the identif i cation and assembly of this small group of patients makes it possible to understand some common rules of clinical operational tolerance in LT as well as possible molecular mechanisms involved. These fi ndings have led some transplant centers to initiate large-scale prospective immunuosuppressant weaning trials.
This kind of clinical research is not easy. Contemporary IS principles have matured over the past three decades and have already yielded great success in clinical transplantations with excellent patient and graft outcomes. Conducting drug weaning/withdrawal trials in otherwise stable patients will undoubtedly face reluctance or resistance from patients as well as their clinicians. It may also carry ethical concerns. In case of failure of tolerance induction, such drug withdrawal may expose patients to rejection, permanent graft damage or even loss. Another major concern is theuncertainty of the stability of the tolerant state since recipients bearing an otherwise mature immune system are constantly exposed to exterior stimuli such as bacterial or viral infections, which can evoke the innate immune system and break the tolerance state. Due to the immunoprivilege as well as strong self-repair and tissue regeneration capacity of the liver allografts, most prospective clinical trials attempting to wean recipients from IS are conducted in liver transplant patients, and they also show the highest success rates. These clinical studies can be divided into two categories based on whether induction therapy was used or not. The time interval between transplantation and drug weaning, the pace of drug weaning, and spaced weaning are the central questions in these trials.

Table 1.Anecdotal evidence of spontaneous operational tolerance in LT
Reduction and withdrawal of IS in patients with stable LT (Table 2)
These patients receive traditional double or triple IS in the early post-operative stage, usually many years after their LT, on minimal dose of immunosuppressive medications and have very stable graft function. Pre-weaning biopsies exclude any signs of rejection, occurrence of original diseases, chronic damage or fi brosis. The transplant team at Kyoto University enrolled 63 stable living donor liver transplantation (LDLT) recipient into the IS weaning trial, including 26 electively weaned and 37 forcibly or incidentally weaned patients due to various causes. The median age at the time of LT was 1.1 years, all patients survived at least 2 years after transplantation with good graft function, andhad no rejection episode in the past year. Twenty-four (38.1%) patients achieved a complete discontinuation of tacrolimus with a median drug-free interval of 23.5 months, 23 (36.5%) are still in the midst of taper and 16 (25.4%) encountered rejection which was readily reversible with reintroduction of IS or steroid bolus.[20]University of California San Francisco (UCSF) recently conducted a very carefully designed, prospective, multicenter pilot study in 20 stable pediatric recipients of parental LDLT for entities other than viral hepatitis or autoimmune diseases. All of them were on a single immunosuppressive drug and had no evidence of rejection or signif i cant fi brosis on pre-weaning biopsies. Notably their median age at the time of transplantation was 6.9 months, and they were at least more than 6 years out of LT at the time of weaning. Gradual IS withdrawal was attempted over a minimum of 36 weeks, recipients were followed up for a median of 32.9 months. Twelve (60%) recipients were found to became clinically tolerant with normal allograft function for a median of 35.7 months after complete cessation of IS. Follow-up biopsies obtained more than 2 years after completing weaning showed no signif i cant change compared with baseline biopsies. Eight patients failed to achieve operational tolerance mainly due to the development of mild acute or indeterminate rejection that were readily reversible. HLA mismatch, sensitization status, and presence of donor specif i c antibodies were not associated with operational tolerance, while increased time interval between transplantation and IS withdrawal was the most important clinical factor associated with successful withdrawal.[28]This important study once again proves that operational tolerance may occur more frequently in children than in adults. The high withdrawal rate in this study may be related to the very young age at the time of transplant (6.9 months), the long interval between the LT and initiation of IS (>6 years and 5 months), and the slow pace of weaning over 36 weeks. Nonetheless, the actual IS withdrawal rate might be similar to the Japanese study because the rate of success in the Kyoto cohort was apparently higher than 38.1% since there were still 36.5% of recipients undergoing weaning. 50%-60% may represent the estimated successful rate of operational tolerance in this highly selected pediatricLDLT population. So far, fewer studies[18,19,24-27]report the incidence of tolerance in the IS weaning trials in adult LT recipients, which varies between 23%-40%. One study sponsored by RISET tested IS reduction and withdrawal in 114 adult patients with stable LT is done at the Digestive Diseases Institute of Barcelona. In another ITN-sponsored study conducted at the University of Pennsylvania, patients with stable liver function who were treated with calcineurin inhibitor (CNI) after LT were randomly assigned to IS withdrawal or maintenance after 12 months.[2]Both trials are still ongoing.
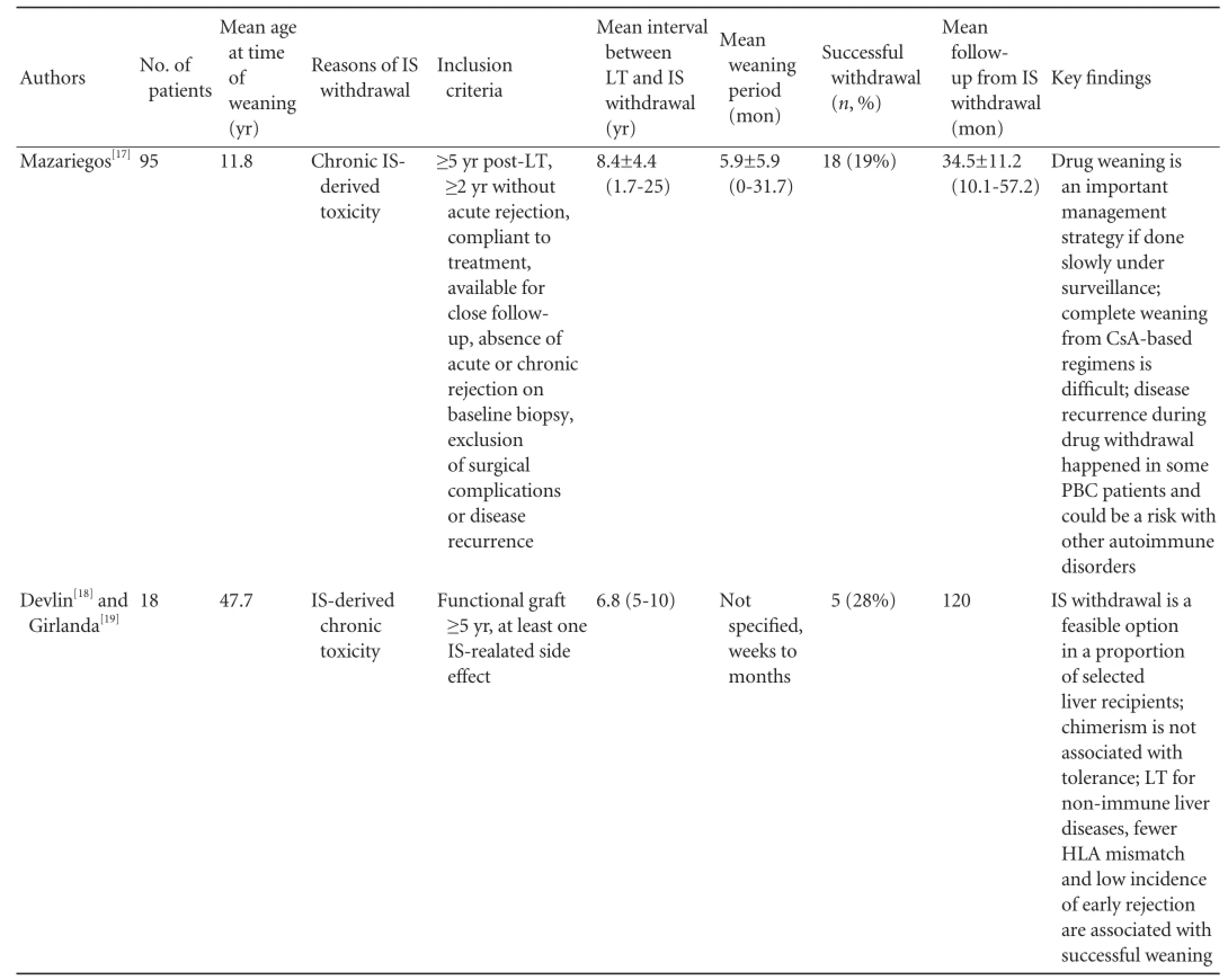
Table 2.Clinical trials of IS weaning under careful monitoring in patients with conventional IS regimen and stable liver graft function

To be continued

ACR: acute cellular rejection; PTLD: post-transplant lymphoproliferative disorder; GVHD: graft-versus-host disease; IS: immunosuppression; N/A: not applicable; Treg: regulatory T cell. Note: several case serials from the same center may be partially overlapped.
Induction therapy followed by maintenance IS reduction or withdrawal (Table 3)
There is much to learn about the precise mechanisms governing the development and maintenance of clinical transplantation tolerance. The current understanding of the central paradigm of transplantation tolerance involves at least two key components: deletion and regulation. First is elimination of a considerable fraction of alloreactive T cells, which then renders the remaining alloreactive T cell repertoire "manageable" by regulatory mechanisms. Campath-1H, the anti-CD52 monoclonal antibody, can eff i ciently and lastingly eliminate human T, B, natural killer (NK) cells and monocytes. Calne et al[35]fi rst used Campath-1H in combination with low-dose CsA in renal transplant recipients, the 5-year patient and graft survival rates of this therapy were similar to the triple immunosuppressive therapy. However, the patients need to be maintained on minimal IS. Campath-1H is incompetent to induce immune tolerance by itself,[36]even used in combination with deoxyspergualin would eventually result in rejection.[37]Subsequent large-scale clinical studies using Campath-1H induction therapy followed by reducing immunosuppressive agents were conducted at the University of Pittsburgh.[29]Ten years' research experience indicated that the effectiveness of this therapy is comparable to traditional high-dose immunosuppressive therapy in LT patients except for hepatitis C,[38]cadaveric and living donor renal transplantations, small intestinal, multivisceral and lung transplantations. Meanwhile, the incidence of opportunistic infections or post-transplantation lymphoproliferative disorders was signif i cantly reduced. Unfortunately, such strategyseems not to be more tolerogenic in comparison with the traditional IS regimen followed by weaning. Based on the preliminary Pittsburgh experience, one ITN-sponsored study was performed at the University of Chicago to reduce or withdraw immunosuppressive agents within 1 to 2 years using Campath-1H induction in combination with FK506 or CsA in LT recipients with non-viral end-stage liver diseases, the tolerance rate has not been reported so far.[2]

Table 3.Clinical trials in operational LT tolerance with induction therapy and/or BM infusion
Immune monitoring in clinical LT tolerance
Effective biomarkers featuring the operationally tolerant liver allograft recipient would allow more successful drug withdrawal with the goal of expanding the potential benef i ts to an increased number of patients. Furthermore, identif i cation of variables predictive of operational tolerance would help to pick out the most suitable patients to enter an IS weaning trial in order to not only improve the success rate but also guarantee the safety and well-being of the patient and graft. More importantly, deciphering the "da Vinci code" of transplantation tolerance would facilitate our understanding of the molecular mechanisms involved and the development of novel therapeutic strategies to achieve better long-term outcomes.
Clinical and immunological monitoring
Implementing drug minimization or withdrawal into clinical routine can only be safely and reliably achieved when guided by means re fl ecting the individual immune condition. It is now well accepted that the laboratory tests routinely performed to assess graft function are not highly sensitive for graft injury. They also play a minimal role in the differential diagnosis of allograft dysfunction since worsening liver function may occur due to factors unrelated to immunological injury (e.g. disease recurrence, viral infections, vascular or biliary complications, etc.). With better understanding of the cellular and molecular mechanisms of transplantation tolerance, novel immunological markers and assays are starting to emerge as invaluable assets for tolerance monitoring (Table 4). In our opinion, although not extensively tested in monitoring operational tolerance, technologies such as trans-vivo delayed type hypersensitivity[8]and ELISPOT assay[53]are among the most promising for transplant tolerance monitoring, as they are capable of measuring the tempo of donor speci fi c immune response.
Pathological monitoring
Although pathological examination has long been recognized as the "golden standard" for the diagnosis of allograft dysfunction, its role in clinical tolerance studies has been largely underestimated or ignored. In the majority of drug weaning trials, biopsies were only performed for graft dysfunction during followup. The current practical guidelines proposed by the Banff expert panels highlight the necessity of preweaning as well as post-weaning protocol biopsies.[54]The pre-weaning biopsies not only document the baseline histological properties of the hepatic allograft for comparison purposes, but also help to exclude those patients with any signs of rejection or fi brosis from the trial. On the other hand, the post-weaning biopsies can help to differentially diagnose subtle allograft pathologies and assist the clinicians with prompt and appropriate decision-making. Another important role to do followup protocol biopsies in patients who are in the process of or after weaning is to gain a better mechanistic insight of clinical LT tolerance, which is still poorly understood at this moment. Although pathological monitoring to determine the cause of graft dysfunction after weaning has becoming an absolute necessity, it is more critical to correlate the histopathologic fi ndings with clinical prof i les to guide the appropriate management and enhance the ability to ultimately wean IS.[5]
Few centers perform follow-up protocol biopsies in order to monitor their IS withdrawal patients. The Kyoto group compared protocol biopsies in pediatric LDLT recipients who had been completely weaned off IS to biopsies from control recipients on maintenance IS. Although graft inf i ltrating Foxp3+ cells were substantially increased, more extensive fi brosis was noted in IS-free recipients, which could be partially resolved or ameliorated after resumption of IS.[55]However, a major confounding factor may be the longer follow-up in the weaning group in this study cohort. In the UCSF study, a group of patients achieved operational tolerance and had less portal inf l ammation and lower total C4d scores on the screening liver biopsy as compared to patients without operational tolerance. Follow-up biopsies showed negligible change of allograft histology more than 2 years after discontinuation of IS.[28]Tisone et al[26]studied the effect of IS weaning in HCV(+) recipients and found that fi brosis did not progress dramatically after weaning or it even regressed. Thus, weaning off IS seems to have had a benef i cial impact on fi brosis progression caused by HCV relapse.
New methods in monitoring transplantation tolerance
There has been an explosive evolution of molecular cell biology and genetics in the past decades. The development of various "-omics" technologies hasenabled us to acquire high throughput data of comprehensive sets of molecules with increasing speed and accuracy. These technologies have been widely exploited in both basic research and molecular diagnostics in clinical practice. Recently, such molecular diagnostic tools have been applied in the monitoring of clinical transplantation tolerance. Panels of genes have been identif i ed as the molecular signature of the tolerance state or the molecular predictor for the development of tolerance. These new weapons in the armamentarium of clinical tolerance monitoring also add novel molecular insight to our understanding of the development and maintenance of the tolerant state.

Table 4.Bioassays for immune monitoring of operational LT tolerance
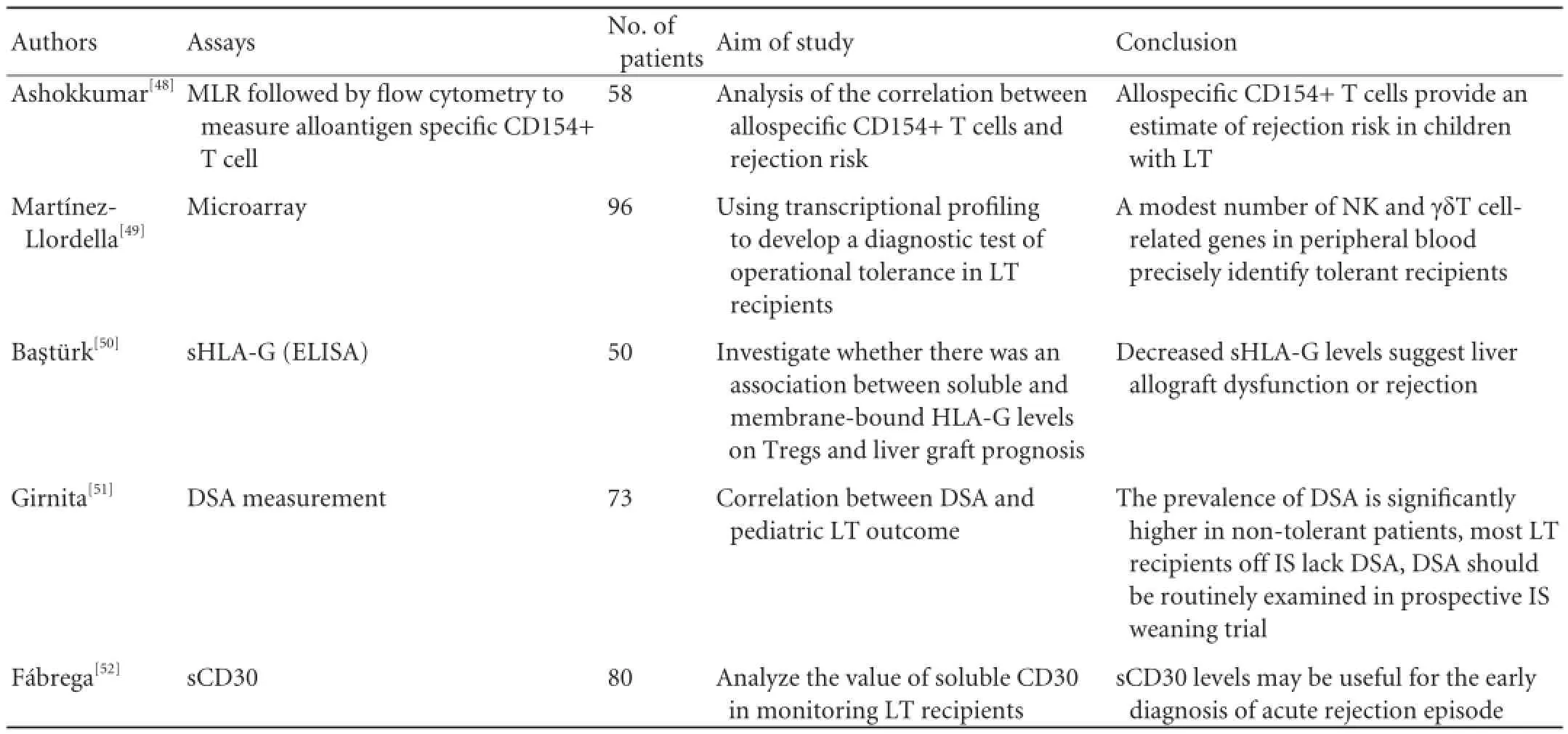
CTL: cytotoxic T lymphocyte assay; DSA: donor-specific HLA antibody; DC: dendritic cells; DTH: delayed-type hypersensitivity: ELISPOT, enzyme-linked immunosorbent spot assay; GrB: granzyme B; IHC: immunohistochemical staining; LT: liver transplantation; mDC: myeloid dendritic cells; MLC: mixed lymphocyte culture; NKT: natural killer T cell; pDC: plasmacytoid dendritic cells; PD-L1: programmed death ligand-1; PW: successful immunosuppression drug weaning; sCD30: soluble CD30; Tc: cytotoxic T cells; Treg: regulatory T cells.
"Biomarker signature" of clinical LT tolerance
Using gene chip technology to select clinical tolerance related markers, two independent studies sponsored by ITN and RISET generated similar fi ndings that tolerant renal transplant recipients highly expressed genes related to B cell differentiation and signaling pathways in their peripheral blood. Adopting similar technology in the tolerant LT patients revealed high expression of genes related to γδT and NK cells. Martinez-Llordella et al[56]compared gene expression prof i les in the peripheral blood of tolerized and nontolerized LT recipients with healthy controls. They found that clinically tolerant patients could be identif i ed not only with a signature of genes encoding several cell surface receptors expressed by NK, CD8+and γδT cells as well as proteins involved in halting cell proliferation, but also by the expansion of CD4+CD25+Foxp3+natural regulatory T cells (nTregs) and γδ TCR+(especially vδ1 TCR+) T cells in the peripheral blood. This genomic and immunological footprint of operational tolerance was subsequently validated in an independent cohort of 23 additional recipients.[49]In a seperate study, transcriptional prof i les were examined by microarrays and quantitative polymerase chain reaction (qPCR) measurements of blood samples from pediatric and adult LT recipients and normal tissues. Tolerancespecif i c genes were validated in independent samples across two different transplant programs. A minimal set of 13 NK cells related genes were signif i cantly expressed in both pediatric and adult tolerant groups, irrespective of different clinical and demographic confounders. The performance of this gene set by microarray showed a sensitivity of 100% and a specif i city of 83%, the area under curve (AUC) was 0.988 for only three genes by qPCR.[21]These results point to a previously unidentif i ed role of NK and γδT cells in LT tolerance. Finally, combination of panels of biomarkers using different assays, the so-called "cross-platform signature", can potentially improve the specif i city and sensitivity of the test with stronger predictive value of operational tolerance. Besides detection of tolerance symbols, biomarkers of acute rejection can alert the clinician tostop the weaning process before parenchymal damage occurs.
Prediction of successful IS weaning
There is no assay available thus far to reliably predict the future development of tolerance, which has more important practical meaning than def i ning a tolerance signature. In a pediatric LT population, Talisetti et al[57]found that variables such as percentage of biliary atresia, type of organ transplanted, history of EB virus infection, donor and recipient gender, and donor-recipient blood type mismatch did not predict the development of tolerance; only age at the time of transplantation was identif i ed as an important predictor of LT tolerance. Ohe et al[58]performed a historic cohort analysis in 134 pediatric primary semi-allogeneic LDLTs at Kyoto University, IS was successfully discontinued in 84 patients. Multivariate logistic regression analysis revealed the absence of early rejection, HLA-A match and the later predominance of Tregs were factors associated with operational tolerance, while HLA-DR mismatches did not affect the development of tolerance. Millán et al[59]noticed even prior to the attempted withdrawal of IS, the peripheral blood cells from the later rejection group produced increased soluble IFNγand displayed a higher percentage of CD8+IL-2+T cells, suggesting that functional differences may be useful to distinguish patients who can be successfully weaned from those who can not. Nadazdin et al[60]suggested that the pretransplant frequency of donor-reactive memory T cells in a given individual may be useful to determine candidacy for tolerance-induction regimens. Besides these clinical and immunological parameters, histological fi ndings in baseline biopsy, such as overall less portal inf i ammation, more regulatory lymphocytes inside the lobules, more advanced portal fi brosis in HCV+ recipients, increased number of Foxp3+ Tregs within the allografts,[61,62]etc, have been proposed to be associated with successful weaning. Conversely, baseline histology in the non-tolerant group exhibited signif i cantly more chronic portal inf i ammation.
Although the previous genetic prof i ling work by the Spanish group can be used as a tool to identify recipients with a tolerant state, a more practical question is how to enroll the most suitable patients into the IS minimization or withdrawal protocol, thus obviating the unnecessary risk of rejection. In a prospective multicenter IS withdrawal trial, Bohne et al[61]analyzed sequential blood and liver tissue specimens collected from LT recipients and compared the gene expression prof i le between tolerant and non-tolerant recipients after a long-term follow-up. Before initiation of drug withdrawal, operationally tolerant and non-tolerant groups differed in the intragraft expression of genes related to iron metabolism; tolerant patients also had higher serum levels of hepcidin and ferritin as well as increased iron deposition within hepatocytes. More importantly, certain hepatic tissue gene expression patterns had a high predictive value of the outcome of IS withdrawal in an independent set of patients. These results suggest a critical role for iron metabolism in the regulation of human intra-graft alloimmune responses and provide a set of biomarkers to enroll the LT patients into drug weaning trials with higher probability of success.
In summary, recently described biomarkers and assays allow identif i cation of patients most suitable for drug weaning or at risk of rejection. The major caveats in all the studies searching for tolerance signatures are insuff i cient patient numbers, uneven clinical data, and different testing methods. Therefore, collaborative efforts are urgently needed to perform well-designed prospective multicenter trials to validate the biomarkers identif i ed across different laboratories. New biotechnologies have also recently been introduced into the study of clinical transplantation tolerance. Genome-wide association (GWA) studies, which use accessible tools for high-throughput genotyping, have now become a standard method for disease gene discovery. At least one multicenter GWA study is being conducted in clinical transplantation (a UK-Irish consortium project funded by the Wellcome Trust Case-Control Consortium) and hopefully will help us gain some preliminary ideas regarding whether the genetic variation in recipients' genomes affects the transplantation outcome.[63]
Impact of IS withdrawal on clinical LT
Few of the studies performed so far have provided a detailed analysis of the impact of IS withdrawal on pre-existing complications derived from the longterm administration of immunosuppressive drugs, they are rather controversial and no solid conclusions can be drawn from these preliminary results. IS withdrawal could be safely attained in selected LT patients and improved not only kidney function but also other cyclosporine associated side-effects such as hypercholesterolemia, hyperuricemia, hypertension, and diabetes.[24]However, Mazariegos et al[17]failed to fi nd any change in renal function or hypertension. Orlando et al[27]examined the effect of IS withdrawal in a cohort of HCV(+) recipients. Initial results showed improvement in fi brosis after withdrawal while no difference was found after a long-term follow-up. Notwithstanding,they did fi nd a decreased incidence ofde novodiabetes, cadiovascular problems and opportunistic infections. Yoshitomi et al[55]reported grafts from operationally tolerant LDLT recipients exhibited more fi brosis, ductular reactions, and decreased luminal diameter of bile ducts as compared with patients under IS treatment, and these abnormalities improved after reintroduction of low-dose IS.
Promising tolerogenic strategies in LT
Mixed chimerism and microchimerism
The hematopoietic chimerism approach stemmed from the original experimental observations of Billingham and Medwar more than half a century ago. The application of "central tolerance" mechanisms by reeducating the recipient immune system is a perfect example of translating a scientif i c idea into clinical practice. Allograft tolerance has been successfully achieved with a non-myeloablative conditioning regimen and donor BMT in human leukocyte antigen (HLA)-matched and mismatched kidney transplantations. The transient mixed chimerism achieved has already resulted in long-term renal allograft tolerance in the majority of patients. The mechanisms of tolerance induction through mixed chimerism may involve central clearance of donor reactive T cells in the thymus (deletion) and regulation (which is manifested by high percentage of Treg subgroups in the peripheral blood and Foxp3/lysosomal enzyme B mRNA in tolerant grafts), etc.[64]The achievement of central tolerance has been a major goal of transplantation research, but in clinical practice, this has been largely impeded by the development of severe graft-versushost disease (GVHD) and serious complications related to recipient pre-conditioning regimens. In several case reports, patients who had received an HLA-identical BMT successfully received subsequent LDLT from the same donor[12,13]or even cadaveric LT,[15]with taper and discontinuation of immunosuppressive therapy. Granot et al[16]reported a patient who had achieved complete chimerism accepted the liver allograft from the same bone marrow donor without any IS after LT. An adolescent patient previously received BMT at the age of 2.5 years from his father, which was later complicated by severe GVHD leading to liver failure and the ensuing LT from the same donor under planned avoidance of IS. Long-term follow-up biopsy did not reveal any sign of rejection. Complete chimerism may also be achieved by BMT after LT. A 4-month-old girl with familial hemophagocytic lymphohistiocytosis underwent stem cell transplantation from her mother 2 months after a LDLT from the same donor for acute hepatic failure. She also received donor lymphocyte infusion on day 43 and the allelic pattern changed to complete donor genotype on day 57. She retained complete donor chimerism and stable hepatic allograft function without any immunosuppressive therapy.[34]To further extend this strategy to more generalized patient population, Donckier et al[31,32]performed rightlobe LDLT after hematological reconstruction following non-myeloablative conditioning and HLA-mismatched stem cell transplantation from the same donor. Totally four out of fi ve patients were able to attain early IS withdrawal, with donor-specif i c hyporesponsiveness in mixed lymphocyte cultures. In the remaining patient, acute rejection developed shortly after discontinuation of IS. Notably, no macrochimerism could be detected in any of the patients during follow-up, once again suggesting the establishment of macrochimerism is not a prerequisite for transplantation tolerance. Interestingly, mixed or full chimerism can be established without the infusion of donor cells under certain circumstances. A 9-year-old female (O, RhD-) patient received urgent LT from a HLA-mismatched 12-year-old male donor O, RhD+ for non-A-to-G hepatitis, and the subsequent development of hemolytic anemia suggested establishment of mixed hematopoietic chimerism. Withdrawal of immunosuppressive therapy resulted in resolution of hemolytic anemia and the development of full hematologic chimerism. The patient remained well 5 years after transplantation. She had been free of any immunosuppressive therapy for 4 years, no liver biopsy was taken. Assessment of antidonor responses in the patient by means of mixedlymphocyte culture 3 years after transplantation showed donor-specif i c unresponsiveness with preserved thirdparty response.[10]The complete absence of GVHD and normal liver function in a fully HLA-mismatched, sexmismatched liver allograft indicate mixed chimerism with full tolerance can occur naturally under certain scenarios.
In the allogeneic transplant setting, microchimerism refers to the presence of a small number of cells that originate from the donor and therefore are genetically distinct from the cells of the host individual. Starzl et al[6,65]observed persistent multilineage hematopoietic cell microchimerism (<1% donor cells) in both lymphoid and nonlymphoid tissues of long-surviving liver or kidney transplantation recipients, including some patients who achieved clinical tolerance. The possible mechanism included the two-way donorrecipient immune interaction and mutual exhaustion of immune reactivity which resulted in clonal deletionof alloreactive cell populations. Tolerogenic donor chimeric cells may also generate regulatory subsets, thus controlling alloimmunity on two fronts. However, it remains controversial over the nature of chimeric cells, their functional signif i cance and the dependence of transplantation tolerance on microchimerism. Recently, it is recognized that the nature of cells that make up the chimerism can inf l uence the graft outcome (rejection versus acceptance).[66]Although the original study indicated that persistent microchimerism may be an important determinant of long-term graft survival and tolerance,[65]subsequent studies clearly challenged this point.[30,67,68]In addition, prospective clinical studies were undertaken to evaluate the potential benef i t of deliberate augmentation of chimerism in conventionally treated liver allograft recipients with donor bone marrow cell infusion.[33]Patients enrolled in the Miami group were at least 3 years post-transplantation with stable graft function. IS was reduced by one third upon enrollment, by another one third the second year of the study and was completely withdrawn the third year. A long-term follow-up after spaced-weaning showed similar survivals of patients and grafts in the two groups. Although rejection episodes were signif i cantly less in the BM infusion group in the fi rst 2 years of the study, there was no signif i cant difference overall. Approximately 20% of long-term survivors of LT were able to successfully discontinue their IS, donor BM infusion did not increase this likelihood. This study clearly showed that co-administration of donor BM cells did not seem to facilitate tolerance.
Regulatory T cells (Tregs)
The past two decades witnessed an explosion in the research of Tregs. Tregs are not only responsible for the control of self-reactive lymphocyte activation and autoimmunity, but also play a key role in dampening the immune responses to pathogens, tumors and allogeneic transplants, thus maintaining the homeostasis of the immune system. Accumulating evidences also support their indispensable role in the induction and maintenance of transplantation tolerance in conjugation with lymphocyte depletion therapies. Harnessing the Tregs to effectively suppress the allo-immune response and to induce tolerance is a very promising strategy in clinical LT. To date, there is no clinical trial in progress which exploits human Tregs for the prolongation of organ transplant survival; however emerging data regarding the safety and eff i cacy of Tregs from clinical BMT trials already provide enough promise to initiate Treg-based therapy in solid organ transplantations. Based on the previous work in rodents, two major strategies have been designed for the therapeutic use of Tregs in humans: adoptive transfer ofex vivoexpanded Tregs andde novoinduction or conversion of non-Tregs cells into Tregsin vivo. The major impediment to Treg cellular therapy is apparently not the safety issue since almost all of the Treg-based clinical trials have shown no infusional toxicity nor increased risk of infection, relapse or early mortality, but rather generating suff i cient numbers of Tregs for therapeutic use. Such need for large numbers of Tregs is based on animal and human study fi ndings that high doses (1:1-1:2 with donor T-cells) were required to maximize eff i cacy.[69]In most phase I/II clinical trials of Tregs in BMT patients, infusion of Tregs was given with doses ranging from 1×106to 3×106/kg, and multiple infusions will most likely be mandatory.[70]It is not clear how much cells are required in the setting of allo-transplantation but they should be no less than the dose used in BMT. Theex vivoexpansion of multilineage nTregs consists of two basic steps: purif i cation of CD4+CD25+nTregs from peripheral blood followed byin vitrostimulation and culture. The current clinical selection protocols for human Tregs still rely on high surface expression of CD25. Human CD4+CD25+Treg population can readily be isolated from peripheral blood using the magnetic column seperation, but only about 60%-70% CD4+25+Foxp3+, indicating that they contain a huge number of effector T cells and can not maintain suff i cient Foxp3 expression or suppressive function when expandedin vitro.[71]In order to improve the purity of nTregs, fl ow cytometry sorting can be applied on the basis of high CD25 expression (top 2%) ± downregulated expression of CD127 (IL-7R a-chain), which is highly expressed by effector and memory T cells. This method can help to generate more than 90% CD4+ Foxp3+ cells which maintain their Foxp3 expression and suppressive function after multiple rounds ofin vitroexpansion. Other than isolation of Tregs from peripheral blood, nTregs can be readily purif i ed from umbilical cord blood (UCB) by taking advantage of the relative paucity of CD25dimnon-Tregs in UCB. Following the selection process, clinically applicable human Tregs can be successfully expanded log-foldin vitrousing bead-bound or artif i cial antigen presenting cell-conjugated anti-CD3 and CD28 antibodies in the presence of high levels of IL-2. Due to the inevitable contamination by non-Treg even with sortor selection, several pharmacological reagents have been exploited to enhance the Treg proliferation or conversion while suppressing the non-Treg division upon TCR ligation and costimulation, such as rapamycin,[72,73]or to increase the stability of nTregs expanded in the presenceof TGF-β[74,75]or rapamycin,[76]like all-trans retinoic acid (ATRA). While a single re-stimulation greatly increases nTreg yield, the trade-off is the time required to generate suff i cient numbers of Tregs for adoptive transfer, which is more problematic for deceased organ transplantation. Creation of nTreg bank is a viable solution.Ex vivoexpanded Tregs can be frozen and stored, which can be used to treat multiple patients after a brief restimulation of aliquots of thawed nTregs.[70]
In addition to expanding naturally-occurring Tregs, much effort has been focused on enhancing Treg eff i cacy by increasingin vivoconversion or their potency.[77]This strategy relies largely upon the different biological properties among various T cell subsets. Pharmacological inhibition of PKC-theta may augment human Treg functionin vitroandin vivo.[78]The Jak-Stat pathway has also been shown to play a critical role in the metabolism of Tregs. Oral Jak inhibitor Tasocitinib (CP-690, 550) effectively can inhibit effector T cell proliferation while sparing the Treg function;[79]augmentation of Stat5 or inhibition of Stat1 signaling has been shown to boost Treg expansion and reduce GVHD lethality in mice.[80,81]Pharmacological inhibition of phosphodiesterase 3 (PDE3) could effectively enhance the enrichment of human CD4+Foxp3+, donor-reactive Tregs driven by allogeneic dendritic cells (DCs) via elevation of cAMP. These adaptive Tregs inhibitin vitroT cell proliferation, and more importantly, attenuated the development of vasculopathy mediated by peripheral blood mononuclear cells in a humanized mouse transplantation model.[82]Epigenetic modif i cation of Treg generation and function has been another interesting research area in the past several years. DNA methyltransferase inhibitor 5-aza-2'-deoxycytidine (Aza) may demethylate the evolutionarily conserved CpG-rich island of the Foxp3 nonintronic upstream enhancer and induce stable Foxp3 expression and suppressive activity.[83]Small molecule inhibitors of another key family of DNA-modifying enzymes histone deacetylases (HDACs) effectively increases Treg numbers and functionin vivoand promote allograft tolerance.[84,85]Generation of donor-specif i c Tregs is an alternative method aside from selection and expansion of polyclonal nTregs. Although they are more potent than the polyclonal Treg population, the yield number for therapeutic purposes remains a major obstacle for future research. Lastly, experimental fi ndings indicate that many immunosuppressive agents can impair the Treg function and thereby transplantation tolerance, indicating that choosing Treg-friendly immunosuppressive regimens to complement the therapeutic strategy may facilitate longterm allograft survival. This will be discussed in details later in this article.
Other cell therapies
Mesenchymal stem cells (MSCs) originally isolated from bone marrow are described as non-hematopoietic, undifferentiated, fi broblast-like and pluripotent progenitor cells. They can differentiate into multiple cell types of mesenchymal origin, such as bone, fat, and cartilage, etc. Phenotypically, they are characterized as high expression of MHC class I molecules, but minimal expression of class II alloantigens and costimulatory molecules; purif i ed T cells do not respond to allogeneic MSCs. MSCs are not only immunoprivileged but display immunoregulatory capacities. They eff i ciently inhibit T cell proliferation, modulate B cell functions, suppress cytotoxic effects of NK cells, as well as the maturation, cytokine production and T cell priming capacity of DCs.[86]Therefore, MSCs can be exploited in clinical immune tolerance. In comparison to the anti-IL-2 receptor antibody induction therapy, Tan et al[87]recently reported that peri-operative infusion of autologous MSCs resulted in a lower incidence of acute rejection and better kidney function at one year among patients who had accepted living donor renal transplantation.
Transplant acceptance-inducing cell (TAIC) is a macrophage subgroup with immune regulation property, it was fi rst discovered in rodent and porcine transplantation models. RISET sponsored the phase I and II clinical trials of inducing renal transplantation tolerance by means of TAIC infusion in combination with reduced immunosuppressive agents. The preliminary outcome showed that TAICs were safe but without tolerogenesis. However, the renal grafts of 4 patients continued to function after FK506 was reduced to low dose and one patient remained off immunosuppressive agents for 8 years. Many patients presented unresponsiveness to donor cells, donor specif i c antibodies could not be detected in all patients.[88,89]At least from the current perspective, strategies exploiting cell therapies do not yield a higher success rate of clinical tolerance. However, their role in LT tolerance needs to be further explored.
Dealing with memory T cells
The presence of allograft specif i c memory T cells in the host before transplantation constitutes a major barrier to transplantation tolerance and further highlights a still unmet need in transplantation medicine. It has been reported that up to 50% of adult human T cell repertoire is composed of memory T cells.[90]The generation of memory T cells is generally thought to be derived from previous infection with pathogens. TCRs recognize sequence specif i c butstructurally similar MHC-peptide complex, therefore many pathogen-specif i c memory T cells are crossreactive with donor allopeptide-MHC complexes, a process sometimes termed "heterologous immunity". Memory T cells are known to be relatively resistant to a variety of therapeutic interventions such as depleting therapies, co-stimulation blockade and suppression by regulatory cells. Induction therapies using aggressive depleting agents like Campath-1 or ATG may favor homeostatic proliferation of memory T cells, therefore paradoxically create a situation which favors allograft rejection and/or a signif i cant barrier for the induction of tolerance.[91,92]Using a non-human primate model, Nadazdin et al[60]suggested that the pre-transplant frequency of donor specif i c memory T cells may be used to determine candidacy for tolerance induction in a given individual. Although tissue typing has rarely been considered necessary in the LT setting given the tolerogenic property of the graft, we may need to reevaluate the importance of HLA match in future clinical tolerance trials and possibly modify our current practice. On the other hand, identif i cation of effective targets and designing new therapeutic strategies to inhibit donorspecif i c memory T cells in transplant recipients may even allow transplantation between donor-recipient pairs with unfavorable memory T cell precursor frequencies. Recent studies[93-95]in animal models have suggested that targeting adhesion molecules such as CD2, leukocyte functional antigen 1 (LFA-1) or very late antigen 4 (VLA-4) can inhibit graft rejection mediated by donor-reactive memory T cells, further clinical trials are warranted to test their roles in the clinical tolerance induction therapies. Apparently, the benef i t gained via attenuating donor-reactive memory T cell responses must be balanced with the cost of dampened protective immunity and potential risk of infection since there is no proven means to accomplish "clone-specif i c" depletion at this stage.
Choosing the optimal tolerogenic IS regimen
Numerous animal and clinical studies[96-117]have investigated the impact of immunotherapeutic regimens on the realization of immune tolerance and the potential mechanisms involved. Since "regulation" is generally considered as the key component of transplantation tolerance, the majority of research have focused on the inf l uence of immunosuppressive agents on Treg population. Lopez et al[96]demonstrated relative expansion of functional CD4+CD25+Foxp3+Tregsin vitrowith ATG at a concentration even 10 times lower than the clinical dosage. Studies in kidney and islet transplant recipients showed that while alemtuzumab increased the relative frequency of Tregs by up to 3 folds, ATG has either a smaller increase or no effect, and use of daclizumab resulted in a decreased Treg population, suggesting alemtuzumab or ATG is the antibody of choice in current clinical practice.[97-100]While most groups consider Campath-1H and ATG as the best depleting antibodies available, it remains controversial as which maintenance immunosuppressant should be chosen in order to promote clinical tolerance. Calcineurin inhibitors (CNIs) such as cyclosporine or tacrolimus are currently the backbone of maintenance IS, and they effectively inhibit the proliferative capacity of allo-responsive T lymphocytes through blockade of the intracellular pathways of T cell activation. However, decreased activation of alloreactive T cells by CNIs can be anti-tolerogenic possibly because of impaired apoptosis. Moreover, accumulating data have suggested that CNIs are detrimental to Tregs. This can be due to reduced Treg induction, expansion and function. Baan et al[101]observed signif i cant inhibition of Foxp3 mRNA amplif i cation by CNIs and anti-CD25 antibodyin vitro.In vivohuman studies also suggested that CNIs lead to lower circulating levels of Tregs than rapamycin[102,103]and CNI tapering[104]despite higher levels than in healthy donors.[105]All these results point to the negative impact of CNIs on the expansion and homeostasis of Tregs. In contrast, rapamycin is generally considered to be tolerance-permissive by selectively inducing apoptosis or necrosis of alloreactive cells whilst promoting Treg induction and expansion as well as sparing the suppressive function. Battaglia et al[106]showed rapamycin selectively expanded the murine nTregsin vitro. Valmori et al[107]demonstrated rapamycin promoted human CD4+regulatory function by inducing suppressive functions in conventional CD4+T cells rather than by selective expansion of nTregs. All these data suggest that rapamycin is a more ideal candidate for maintenance IS than CNI. Such Treg-friendly effect might be attributed to the inhibition of the PI3K/Akt/ mTOR signaling pathway by the constitutively expressed phosphatase and tensin homolog (PTEN) in Tregs and the relative dominance of the JAK/STAT/Pim-2 pathway in Tregs compared to conventional T cells.[108,109]In Tregs, Pim-2 expression is controlled by Foxp3, and it is constitutively expressed to offer an alternative pathway rendering the PI3K/Akt/mTOR pathway dispensable for Treg function and survival. Tregs are also relatively resistant to apoptosis compared with Tconv after treatment with rapamycin.[110]Besides Tregs, CNIs and rapamycin may have differed effect on DC function.[111]A recent study described an unexpected property of rapamycin which selectively boostered the anti-pathogenCD8+T cell reaction but not the allograft specif i c cytotoxic T cell response.[112]In spite of all these animal studies showing that CNIs might be anti-tolerogenic while rapamycin is tolerance-permissive, and rapamycin but not CNIs can enhance the human Treg population, conversion, function or survival, we should never ignore the fact such superiority has never been proved in clinical tolerance studies. One of the major concerns of using rapamycin as the primary immunosuppresive agent is that it is not as potent as CNIs in dampening the allogeneic immune responses. Whether additional IS, for example CNIs or MMF will be required in the short-term remains an interesting topic. In an ITN sponsored study, the research team from the University of Wisconsin made use of Campath-1H in combination with rapamycin to treat renal transplantation patients. FK506 was added to the rapamycin for 60 days postoperatively followed by rapamycin monotherapy and drug weaning to prevent acute rejection. 90% of the patients on low dose rapamycin remained favorable renal function.[113]Another limitation can be the relative ineff i cacy of rapamycin to inhibit the proliferation and function of memory T cells, which are sensitive to CNIs.[114]Therefore, future clinical trials are def i nitely needed to conf i rm the superiority of rapamycin in clinical transplantation tolerance. The inf l uence on Treg function and tolerance by other IS medications have also been studied. Corticosteroids do not inhibit Tregs and may even promote their expansion. Mycophenolate mofetil (MMF) treatment increased rodent allograft survival and the Treg population in recipient secondary lymphoid organs.[115]After conversion from cyclosporine to MMF, LT patients had higher numbers of circulating Tregs,[116]although no direct expansion of mouse Tregs with MMF was observedin vitro.[117]Finally, not only was Treg sequestration differentially affected but Treg activity was enhanced in the presence of FTY720in vitro.[118]Whether new generation of biological agents such as costimulatory blocker Belatacept or various chemokine antagonists facilitate tolerance induction and/or maintenance remains an important topic in future clinical trials.
Innate immunity and LT tolerance
It is not until recently that the role of innate immunity in transplant tolerance has been appreciated. The innate immune system acts as the frontline of defense against pathogens or noxious stimuli. These stimuli activate the pattern recognition receptor (PRR) family members expressed on immune cells. Toll-like receptors (TLRs) are the best characterized PRRs mainly located on the plasma membrane. They primarily recognize bacterial motifs (TLR1, 2, 4, 5, 6, and 11). There is also another subset of TLRs expressed in endosomal compartments that recognize nucleic acids specif i c for viral recognition (TLR3, 7, and 8) or unmethylated CpG motifs common to both viruses and bacteria (TLR9).[119]Ligation of TLRs on antigen presenting cells (APCs) promotes their survival, activates downstream signaling events and adaptive immune responses.[120]The roles of toll-like receptors (TLRs) in both experimental models of acute allograft rejection and transplant tolerance in addition to clinical studies of organ transplantation have also been reported. There are several other classes of PRRs including Nod-like receptors (NLRs), RIG-I—like receptors (RLRs), and C-type lectin receptors (CLRs) which play critical roles in the antimicrobial immunity. Signaling via all these innate immune receptors induces an inf l ammatory program consisting of the production of proinf l ammatory cytokines, such as IL-6 and TNF-α, and the upregulation of costimulatory molecules and chemokines, which results in DC maturation, migration and activation of the adaptive immune responses. Solid evidence from both experimental and clinical studies has suggested that bacterial and viral infections stimulate innate immunity, which can qualitatively and quantitatively alter the magnitude of the alloreactive immune response, thereby not only triggering allograft rejection but also preventing the induction of transplantation tolerance or reversing the existing tolerant state.[121]Established tolerance may be more diff i cult to abrogate since signif i cantly more pro-inf l ammatory signals are required to reverse the stable tolerance state.[122]Besides microbial infections, endogenous molecules are an equally important cause leading to activation of the innate immunity. Damageassociated molecular patterns (DAMPs), or alarmins, are molecules released by damaged, necrotic or stressed cells associated with an inf l ammatory response. DAMPs such as high mobility group box 1 (HMGB1), uric acid, and heat shock proteins serve as endogenous danger signals or ligands, engage several stress receptors like receptor for advanced glycation end products, triggering receptor expressed on myeloid cells-1 and certain TLRs to activate the innate immune response. In the LT setting, ischemia-reperfusion injury (IRI) inevitably leads to the release of endogenous innate immune activators. Some of them are known to activate TLRs (eg, TLR4). In a mouse model, TLR4 but not TLR2 def i ciency conveyed protection from hepatic IRI.[123,124]
The understanding of mechanisms involved in the impairment of immune tolerance upon activation of the innate immune system has just begun to be elucidated.Certain pro-inf l ammatory cytokines such as IL-6, TNF-α and type I interferons induced by PRRs can augment alloreactive T cell responses and render these cells resistant to immune regulation. They also impair the ability of donor-specif i c Tregs to suppress effector T cell response by destabilizing Treg immunoregulatory phenotype, thus preventing transplant tolerance induction.[125,126]Interventions that dampen adverse inf l ammatory responses may help to create a milieu that guides many donor reactive T cells into a tissueprotective phenotype, and promote graft acceptance or even tolerance. Blockade of certain proinf l ammatory cytokines such as IL-1, TNF-α and IL-6, supplementation with acute phase proteins like alpha 1-antitrypsin (α1-AT) and C1-esterase inhibitor, enhancing the expression of tissue-intrinsic cytoprotective genes (HO-1, A20, bcl-2, bcl-xl), transcription factors (HIF-1α) or certain metabolites (carbon monoxide, adenosine) are among the measures to ameliorate IRI of the liver allograft.[127]It may be worth conducting clinical trials to test whether dampening the innate immunity would reduce the overall requirements for maintenance IS and possibly facilitate transplant tolerance.
Discussion
The current standard care for transplant recipients is largely a one-size-f i ts-all approach to IS. Regimens are frequently only modif i ed as a response to acute rejection episodes, serious infectious complications or toxicities. The long-term side effects with a substantial cost to the patients have rendered researchers to seek alternative strategies. Immune tolerance has long been regarded as the "holy grail" of transplantation medicine, on which many clinicians and immunologists have made indefatigable efforts. In the past two decades, accumulating evidence suggests that clinical operational tolerance is rare but achievable after organ transplantation. The long-term aim has been realized in some patients with liver or renal transplantations via developing mixed chimera or antibody induction therapies followed by stepwise IS weaning under strict monitoring. Some patients are also identif i ed to have developed spontaneous operational tolerance due to non-compliance. Signif i cant differences exist between tolerant recipients of different organs. Lessons learned from tolerance observed in one type of organ transplant may not be directly applied to others. The robust immune response and inf l ammation in the early post-transplant stage probably explain why more IS is needed to prevent rejection and why it is more diff i cult to wean IS at this time. It also provides the rationale to use lymphocyte depletion agents in tolerogenic therapies. Importantly in the LT setting, the onset of an episode of acute rejection during the attempt of IS withdrawal will not worsen the later clinical outcome. It renders LT recipients more willing to be enrolled into weaning trials. Molecule-based regimens have been largely unsuccessful given the complexity of human immune system, but cell-based tolerance protocols have delivered some encouraging results to achieve clinical operational tolerance. With the establishment of ITN and RISET consortia, intense efforts have been made to conduct well-designed and carefully monitored clinical trials to elucidate the related mechanisms and search for molecular signatures of transplantation tolerance. Some γδT and NK cell-related molecules can potentially become biomarkers for operational LT tolerance. Other molecules pertaining to iron metabolism have been found to be predictive of successful IS withdrawal.
Despite all the progress, the study of clinical tolerance is still in its infancy. We may only be seeing the tip of the iceberg, and there is a long road ahead to develop stable and highly reproducible immune tolerance for clinical application. Given the major difference between rodents and humans, the many tolerance induction protocols used in rodent animal models have been largely unsuccessful in non-human primates and clinical studies, mainly due to the presence of signif i cant numbers of memory T cells. Tolerance is not yet reliably achieved in most human organ transplantation settings. There is still lack of consensus regarding the def i nition and mechanisms of operational tolerance. The same applies to tolerance induction methods. Meanwhile, strategies using IS weaning with or without depletion antibody to achieve tolerance is rather "passive". There is no solid evidence to support that antibody induction together with IS minimization is superior to conventional IS with regard to the later tolerance.[128]
The key to a potential major breakthrough in clinical transplantation tolerance relies largely on basic research to further clarify the mechanisms of tolerance. On the other hand, we should possibly focus more on learning the mechanisms of clinical operational tolerance and design new therapeutic strategies. All tolerance protocols learned from animal studies should be carefully tested and modif i ed in large animal or human studies. We propose several potential tolerance induction strategies in the current review, including reprogramming or reeducating the recipient immune system by establishing mixed chimerism, Treg-based therapy, cell therapy with MSCs or TAICs and overcoming the hurdle of T cell memory and control of innate immunity. Althoughstrategies using co-stimulation blockade or immature DCs which showed great tolerogenic potential in animal studies are not included, we should not underestimate their role in the process of immune tolerance. However, since memory T cells are largely insensitive to costimulation blockade and also because we currently have no reliable means to control the maturation of "tolerogenic" DCs upon stimulation with external or internal stimuli via the PRRs or upon DC-T interactions, their role in clinical tolerance awaits further proof. With regard to clinical tolerance studies, the continuous identif i cation of those individuals who developed spontaneous LT tolerance is not an easy task. Therefore, tolerance registries and multicenter studies are needed for to include clinically meaningful numbers. Current biomarkers need to be validated in large-scale clinical trials. New methods with high sensitivity and specif i city to predict the likelihood of tolerance will have to be developed. Integrated clinical, biological and pathological monitoring of the weaning recipients is mandatory to decrease the risk of graft damage or loss in case of failure of tolerance.
Another major issue in clinical tolerance trials is inclusion criteria. The majority of clinical tolerance trials so far have excluded patients whose primary diseases are viral hepatitis or immune-mediated hepatic diseases, due to concerns of worsening primary illness or disease recurrence. Since these two major categories of liver diseases are the main indications for LT, everywhere, excluding these patients from clinical tolerance trials will prevent a substantial number of recipients from achieving an IS-free state. Given that preliminary clinical data showed that IS weaning in HCV patients does not worsen the outcome but it may slow down the progression of allograft fi brosis, and that viral hepatitis patients may benef i t from minimization of IS, we should include this large patient population in future clinical trials. Adjustment of the lymphocyte depletion antibody dose may be still required. For patients with immune-mediated liver diseases, there is no solid evidence to support that reduction or withdrawal of IS will increase recurrence of the primary diseases, except for some primary biliary cirrhosis cases. Tolerogenic strategies such as targeting the memory T cell population and antigen non-specif i c Treg therapy may help to control the autoimmune T cell clones and mitigate disease recurrence. Regarding the most widely used "spaced weaning" method, it may be worthwhile to determine the optimal interval from transplant to the start of IS weaning, the pace of weaning and the duration from the start of IS minimization to complete withdrawal and the essential follow-up period. With more intimate collaborative efforts from the researchers, clinical physicians and pharmaceutical companies, intellectual and fi nancial resources can be synergized towards the ultimate goal of clinical tolerance. Stable operational tolerance is expected to be realized in more generalized clinical LT recipients, perhaps even in the near future.
Contributors:TR proposed the study, retrieved and summarized the relevant literatures. LXQ, HZQ and TR wrote the main body of the article. TR revised the review and is the guarantor.
Funding:This work was supported by grants from National Science Foundation (81001324), PhD site special research grant from Ministry of Education (20100073120094) and Endowed Professorship ("Oriental Scholar") funding from Shanghai Municipal Science and Technology Committee.
Ethical approval:Not needed.
Competing interest:No benef i ts in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Calne R, Watson CJ. Some observations on prope tolerance. Curr Opin Organ Transplant 2011;16:353-358.
2 Turka LA, Wood K, Bluestone JA. Bringing transplantation tolerance into the clinic: lessons from the ITN and RISET for the establishment of tolerance consortia. Curr Opin Organ Transplant 2010;15:441-448.
3 Bluestone JA, Auchincloss H, Nepom GT, Rotrosen D, St Clair EW, Turka LA. The Immune Tolerance Network at 10 years: tolerance research at the bedside. Nat Rev Immunol 2010;10:797-803.
4 Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev 2006;213:101-118.
5 Demetris AJ, Lunz JG 3rd, Randhawa P, Wu T, Nalesnik M, Thomson AW. Monitoring of human liver and kidney allograft tolerance: a tissue/histopathology perspective. Transpl Int 2009;22:120-141.
6 Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology 1993;17:1127-1152.
7 Reyes J, Zeevi A, Ramos H, Tzakis A, Todo S, Demetris AJ, et al. Frequent achievement of a drug-free state after orthotopic liver transplantation. Transplant Proc 1993;25:3315-3319.
8 VanBuskirk AM, Burlingham WJ, Jankowska-Gan E, Chin T, Kusaka S, Geissler F, et al. Human allograft acceptance is associated with immune regulation. J Clin Invest 2000;106: 145-155.
9 Hsu LW, Goto S, Nakano T, Lai CY, Lin YC, Kao YH, et al. Immunosuppressive activity of serum taken from a liver transplant recipient after withdrawal of immunosuppressants. Transpl Immunol 2007;17:137-146.
10 Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, et al. Chimerism and tolerance in a recipient of a deceaseddonor liver transplant. N Engl J Med 2008;358:369-374.
11 Gras J, Wieërs G, Vaerman JL, Truong DQ, Sokal E, Otte JB, et al. Early immunological monitoring after pediatric liver transplantation: cytokine immune deviation and graft acceptance in 40 recipients. Liver Transpl 2007;13:426-433.
12 Kadry Z, Mullhaupt B, Renner EL, Bauerfeind P, Schanz U, Pestalozzi BC, et al. Living donor liver transplantation and tolerance: a potential strategy in cholangiocarcinoma. Transplantation 2003;76:1003-1006.
13 Andreoni KA, Lin JI, Groben PA. Liver transplantation 27 years after bone marrow transplantation from the same living donor. N Engl J Med 2004;350:2624-2625.
14 Mellgren K, Fasth A, Saalman R, Olausson M, Abrahamsson J. Liver transplantation after stem cell transplantation with the same living donor in a monozygotic twin with acute myeloid leukemia. Ann Hematol 2005;84:755-757.
15 Urban CH, Deutschmann A, Kerbl R, Lackner H, Schwinger W, Königsrainer A, et al. Organ tolerance following cadaveric liver transplantation for chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transplant 2002;30:535-537.
16 Granot E, Loewenthal R, Jakobovich E, Gazit E, Sokal E, Reding R. Living related liver transplant following bone marrow transplantation from same donor: long-term survival without immunosuppression. Pediatr Transplant 2012;16:E1-4.
17 Mazariegos GV, Reyes J, Marino IR, Demetris AJ, Flynn B, Irish W, et al. Weaning of immunosuppression in liver transplant recipients. Transplantation 1997;63:243-249.
18 Devlin J, Doherty D, Thomson L, Wong T, Donaldson P, Portmann B, et al. Def i ning the outcome of immunosuppression withdrawal after liver transplantation. Hepatology 1998;27:926-933.
19 Girlanda R, Rela M, Williams R, O'Grady JG, Heaton ND. Long-term outcome of immunosuppression withdrawal after liver transplantation. Transplant Proc 2005;37:1708-1709.
20 Takatsuki M, Uemoto S, Inomata Y, Egawa H, Kiuchi T, Fujita S, et al. Weaning of immunosuppression in living donor liver transplant recipients. Transplantation 2001;72:449-454.
21 Li Y, Koshiba T, Yoshizawa A, Yonekawa Y, Masuda K, Ito A, et al. Analyses of peripheral blood mononuclear cells in operational tolerance after pediatric living donor liver transplantation. Am J Transplant 2004;4:2118-2125.
22 Koshiba T, Li Y, Takemura M, Wu Y, Sakaguchi S, Minato N, et al. Clinical, immunological, and pathological aspects of operational tolerance after pediatric living-donor liver transplantation. Transpl Immunol 2007;17:94-97.
23 Pons JA, Yélamos J, Ramírez P, Oliver-Bonet M, Sánchez A, Rodríguez-Gago M, et al. Endothelial cell chimerism does not inf l uence allograft tolerance in liver transplant patients after withdrawal of immunosuppression. Transplantation 2003;75:1045-1047.
24 Pons JA, Ramírez P, Revilla-Nuin B, Pascual D, Baroja-Mazo A, Robles R, et al. Immunosuppression withdrawal improves long-term metabolic parameters, cardiovascular risk factors and renal function in liver transplant patients. Clin Transplant 2009;23:329-336.
25 Pons JA, Revilla-Nuin B, Baroja-Mazo A, Ramírez P, Martínez-Alarcón L, Sánchez-Bueno F, et al. FoxP3 in peripheral blood is associated with operational tolerance in liver transplant patients during immunosuppression withdrawal. Transplantation 2008;86:1370-1378.
26 Tisone G, Orlando G, Cardillo A, Palmieri G, Manzia TM, Baiocchi L, et al. Complete weaning off immunosuppression in HCV liver transplant recipients is feasible and favourably impacts on the progression of disease recurrence. J Hepatol 2006;44:702-709.
27 Orlando G, Manzia T, Baiocchi L, Sanchez-Fueyo A, Angelico M, Tisone G. The Tor Vergata weaning off immunosuppression protocol in stable HCV liver transplant patients: the updated follow up at 78 months. Transpl Immunol 2008; 20:43-47.
28 Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA 2012;307: 283-293.
29 Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, et al. Tolerogenic immunosuppression for organ transplantation. Lancet 2003;361:1502-1510.
30 Eason JD, Cohen AJ, Nair S, Alcantera T, Loss GE. Tolerance: is it worth the risk? Transplantation 2005;79:1157-1159.
31 Donckier V, Troisi R, Toungouz M, Colle I, Van Vlierberghe H, Jacquy C, et al. Donor stem cell infusion after nonmyeloablative conditioning for tolerance induction to HLA mismatched adult living-donor liver graft. Transpl Immunol 2004;13:139-146.
32 Donckier V, Troisi R, Le Moine A, Toungouz M, Ricciardi S, Colle I, et al. Early immunosuppression withdrawal after living donor liver transplantation and donor stem cell infusion. Liver Transpl 2006;12:1523-1528.
33 Tryphonopoulos P, Tzakis AG, Weppler D, Garcia-Morales R, Kato T, Madariaga JR, et al. The role of donor bone marrow infusions in withdrawal of immunosuppression in adult liver allotransplantation. Am J Transplant 2005;5:608-613.
34 Matthes-Martin S, Peters C, Königsrainer A, Fritsch G, Lion T, Heitger A, et al. Successful stem cell transplantation following orthotopic liver transplantation from the same haploidentical family donor in a girl with hemophagocytic lymphohistiocytosis. Blood 2000;96:3997-3999.
35 Calne R, Friend P, Moffatt S, Bradley A, Hale G, Firth J, et al. Prope tolerance, perioperative campath 1H, and low-dose cyclosporin monotherapy in renal allograft recipients. Lancet 1998;351:1701-1702.
36 Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specif i c monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation 2003;76:120-129.
37 Kirk AD, Mannon RB, Kleiner DE, Swanson JS, Kampen RL, Cendales LK, et al. Results from a human renal allograft tolerance trial evaluating T-cell depletion with alemtuzumab combined with deoxyspergualin. Transplantation 2005;80: 1051-1059.
38 Marcos A, Eghtesad B, Fung JJ, Fontes P, Patel K, Devera M, et al. Use of alemtuzumab and tacrolimus monotherapy for cadaveric liver transplantation: with particular reference to hepatitis C virus. Transplantation 2004;78:966-971.
39 Truong DQ, Cornet A, Wieërs G, Robert A, Reding R, Latinne D. Pre- and post-transplant monitoring of granzyme B enzyme-linked immunosorbent spot assay in pediatric liver recipients. Transpl Immunol 2008;19:215-219.
40 Stenard F, Nguyen C, Cox K, Kambham N, Umetsu DT, Krams SM, et al. Decreases in circulating CD4+CD25hiFOXP3+cells and increases in intragraft FOXP3+ cells accompany allograft rejection in pediatric liver allograft recipients. Pediatr Transplant 2009;13:70-80.
41 Israeli M, Klein T, Sredni B, Avitzur Y, Mor E, Bar-Nathen N, et al. ImmuKnow: a new parameter in immune monitoring of pediatric liver transplantation recipients. Liver Transpl 2008;14:893-898.
42 Mazariegos GV, Zahorchak AF, Reyes J, Chapman H, Zeevi A, Thomson AW. Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. Am J Transplant 2005;5:314-322.
43 Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, et al. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant 2003;3:689-696.
44 Tokita D, Mazariegos GV, Zahorchak AF, Chien N, Abe M, Raimondi G, et al. High PD-L1/CD86 ratio on plasmacytoid dendritic cells correlates with elevated T-regulatory cells in liver transplant tolerance. Transplantation 2008;85:369-377.
45 Mazariegos GV, Reyes J, Webber SA, Thomson AW, Ostrowski L, Abmed M, et al. Cytokine gene polymorphisms in children successfully withdrawn from immunosuppression after liver transplantation. Transplantation 2002;73:1342-1345.
46 Gupta A, Kumar CA, Ningappa M, Sun Q, Higgs BW, Snyder S, et al. Elevated myeloid: plasmacytoid dendritic cell ratio associates with late, but not early, liver rejection in children induced with rabbit anti-human thymocyte globulin. Transplantation 2009;88:589-594.
47 Geissler F, Jankowska-Gan E, DeVito-Haynes LD, Rhein T, Kalayoglu M, Sollinger HW, et al. Human liver allograft acceptance and the "tolerance assay":in vitroanti-donor T cell assays show hyporeactivity to donor cells, but unlike DTH, fail to detect linked suppression. Transplantation 2001; 72:571-580.
48 Ashokkumar C, Talukdar A, Sun Q, Higgs BW, Janosky J, Wilson P, et al. Allospecif i c CD154+ T cells associate with rejection risk after pediatric liver transplantation. Am J Transplant 2009;9:179-191.
49 Martínez-Llordella M, Lozano JJ, Puig-Pey I, Orlando G, Tisone G, Lerut J, et al. Using transcriptional prof i ling to develop a diagnostic test of operational tolerance in liver transplant recipients. J Clin Invest 2008;118:2845-2857.
50 Baştürk B, Karakayali F, Emiroğlu R, Sözer O, Haberal A, Bal D, et al. Human leukocyte antigen-G, a new parameter in the follow-up of liver transplantation. Transplant Proc 2006;38:571-574.
51 Girnita A, Mazariegos GV, Castellaneta A, Reyes J, Bentlejewski C, Thomson AW, et al. Liver transplant recipients weaned off immunosuppression lack circulating donor-specif i c antibodies. Hum Immunol 2010;71:274-276.
52 Fábrega E, Unzueta MG, Cobo M, Casafont F, Amado JA, Romero FP. Value of soluble CD30 in liver transplantation. Transplant Proc 2007;39:2295-2296.
53 Dinavahi R, Heeger PS. T-cell immune monitoring in organ transplantation. Curr Opin Organ Transplant 2008;13:419-424.
54 Banff Working Group on Liver Allograft Pathology. Importance of liver biopsy fi ndings in immunosuppression management: biopsy monitoring and working criteria for patients with operational tolerance. Liver Transpl 2012;18: 1154-1170.
55 Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation 2009;87:606-614.
56 Martínez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, et al. Multiparameter immune prof i ling of operational tolerance in liver transplantation. Am J Transplant 2007;7:309-319.
57 Talisetti A, Hurwitz M, Sarwal M, Berquist W, Castillo R, Bass D, et al. Analysis of clinical variables associated with tolerance in pediatric liver transplant recipients. Pediatr Transplant 2010;14:976-979.
58 Ohe H, Waki K, Yoshitomi M, Morimoto T, Nafady-Hego H, Satoda N, et al. Factors affecting operational tolerance after pediatric living-donor liver transplantation: impact of early post-transplant events and HLA match. Transpl Int 2012;25:97-106.
59 Millán O, Benitez C, Guillén D, López A, Rimola A, Sánchez-Fueyo A, et al. Biomarkers of immunoregulatory status in stable liver transplant recipients undergoing weaning of immunosuppressive therapy. Clin Immunol 2010;137:337-346.
60 Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells inf l uence tolerance to kidney allografts in nonhuman primates. Sci Transl Med 2011;3:86ra51.
61 Bohne F, Martínez-Llordella M, Lozano JJ, Miquel R, Benítez C, Londoño MC, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest 2012;122:368-382.
62 Yoshitomi M, Koshiba T, Haga H, Li Y, Zhao X, Cheng D, et al. Requirement of protocol biopsy before and after complete cessation of immunosuppression after liver transplantation. Transplantation 2009;87:606-614.
63 Naesens M, Sarwal MM. Molecular diagnostics in transplantation. Nat Rev Nephrol 2010;6:614-628.
64 Sachs DH, Sykes M, Kawai T, Cosimi AB. Immunointervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol 2011;23:165-173.
65 Starzl TE, Demetris AJ, Murase N, Ildstad S, Ricordi C, Trucco M. Cell migration, chimerism, and graft acceptance. Lancet 1992;339:1579-1582.
66 Mathew JM, Leventhal JR, Miller J. Microchimerism in promoting graft acceptance in clinical transplantation. Curr Opin Organ Transplant 2011;16:345-352.
67 Schlitt HJ, Hundrieser J, Ringe B, Pichlmayr R. Donor-type microchimerism associated with graft rejection eight years after liver transplantation. N Engl J Med 1994;330:646-647.
68 Schlitt HJ. Is microchimerism needed for allograft tolerance? Transplant Proc 1997;29:82-84.
69 Bluestone JA, Tang Q, Sedwick CE. T regulatory cells in autoimmune diabetes: past challenges, future prospects. J Clin Immunol 2008;28:677-684.
70 Hippen KL, Riley JL, June CH, Blazar BR. Clinical perspectives for regulatory T cells in transplantation tolerance. Semin Immunol 2011;23:462-468.
71 Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH, Blazar BR, et al.In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood 2004;104:453-461.
72 Hippen KL, Merkel SC, Schirm DK, Nelson C, TennisNC, Riley JL, et al. Generation and large-scale expansion of human inducible regulatory T cells that suppress graftversus-host disease. Am J Transplant 2011;11:1148-1157.
73 Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, et al. Massiveex vivoexpansion of human natural regulatory T cells (T(regs)) with minimal loss ofin vivofunctional activity. Sci Transl Med 2011;3:83ra41.
74 Wang J, Huizinga TW, Toes RE. De novo generation and enhanced suppression of human CD4+CD25+ regulatory T cells by retinoic acid. J Immunol 2009;183:4119-4126.
75 Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-β and retinoic acid. PLoS One 2010;5:e15150.
76 Golovina TN, Mikheeva T, Brusko TM, Blazar BR, Bluestone JA, Riley JL. Retinoic acid and rapamycin differentially affect and synergistically promote theex vivoexpansion of natural human T regulatory cells. PLoS One 2011;6:e15868.
77 Sagoo P, Lombardi G, Lechler RI. Regulatory T cells as therapeutic cells. Curr Opin Organ Transplant 2008;13:645-653.
78 Zanin-Zhorov A, Ding Y, Kumari S, Attur M, Hippen KL, Brown M, et al. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science 2010;328:372-376.
79 Sewgobind VD, Quaedackers ME, van der Laan LJ, Kraaijeveld R, Korevaar SS, Chan G, et al. The Jak inhibitor CP-690,550 preserves the function of CD4CD25FoxP3 regulatory T cells and inhibits effector T cells. Am J Transplant 2010;10:1785-1795.
80 Vogtenhuber C, Bucher C, Highf i ll SL, Koch LK, Goren E, Panoskaltsis-Mortari A, et al. Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood 2010;116:466-474.
81 Ma H, Lu C, Ziegler J, Liu A, Sepulveda A, Okada H, et al. Absence of Stat1 in donor CD4+T cells promotes the expansion of Tregs and reduces graft-versus-host disease in mice. J Clin Invest 2011;121:2554-2569.
82 Feng G, Nadig SN, Bäckdahl L, Beck S, Francis RS, Schiopu A, et al. Functional regulatory T cells produced by inhibiting cyclic nucleotide phosphodiesterase type 3 prevent allograft rejection. Sci Transl Med 2011;3:83ra40.
83 Lal G, Zhang N, van der Touw W, Ding Y, Ju W, Bottinger EP, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol 2009;182:259-273.
84 Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 2007;13:1299-1307.
85 Akimova T, Ge G, Golovina T, Mikheeva T, Wang L, Riley JL, et al. Histone/protein deacetylase inhibitors increase suppressive functions of human FOXP3+ Tregs. Clin Immunol 2010;136:348-363.
86 Wu XB, Tao R. Hepatocyte differentiation of mesenchymal stem cells. Hepatobiliary Pancreat Dis Int 2012;11:360-371.
87 Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA 2012;307:1169-1177.
88 Hutchinson JA, Brem-Exner BG, Riquelme P, Roelen D, Schulze M, Ivens K, et al. A cell-based approach to the minimization of immunosuppression in renal transplantation. Transpl Int 2008;21:742-754.
89 Hutchinson JA, Riquelme P, Brem-Exner BG, Schulze M, Matthäi M, Renders L, et al. Transplant acceptanceinducing cells as an immune-conditioning therapy in renal transplantation. Transpl Int 2008;21:728-741.
90 Ford ML, Larsen CP. Transplantation tolerance: memories that haunt us. Sci Transl Med 2011;3:86ps22.
91 Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memorylike phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 2005;5: 465-474.
92 Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant 2012;12:1079-1090.
93 Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes costimulation blockade based allograft survival in nonhuman primates. Nat Med 2009;15:746-749.
94 Badell IR, Russell MC, Thompson PW, Turner AP, Weaver TA, Robertson JM, et al. LFA-1-specif i c therapy prolongs allograft survival in rhesus macaques. J Clin Invest 2010;120:4520-4531.
95 Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant 2012;12:69-80.
96 Lopez M, Clarkson MR, Albin M, Sayegh MH, Najaf i an N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol 2006;17:2844-2853.
97 Bestard O, Cruzado JM, Mestre M, Caldés A, Bas J, Carrera M, et al. Achieving donor-specif i c hyporesponsiveness is associated with FOXP3+ regulatory T cell recruitment in human renal allograft inf i ltrates. J Immunol 2007;179:4901-4909.
98 Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, et al. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant 2008;8:793-802.
99 Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation 2005;80:457-465.
100 Toso C, Edgar R, Pawlick R, Emamaullee J, Merani S, Dinyari P, et al. Effect of different induction strategies on effector, regulatory and memory lymphocyte subpopulations in clinical islet transplantation. Transpl Int 2009;22:182-191.
101 Baan CC, van der Mast BJ, Klepper M, Mol WM, Peeters AM, Korevaar SS, et al. Differential effect of calcineurin inhibitors, anti-CD25 antibodies and rapamycin on the induction of FOXP3 in human T cells. Transplantation 2005;80:110-117.
102 Segundo DS, Ruiz JC, Izquierdo M, Fernández-Fresnedo G, Gómez-Alamillo C, Merino R, et al. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplantrecipients. Transplantation 2006;82:550-557.
103 Levitsky J, Miller J, Wang E, Rosen A, Flaa C, Abecassis M, et al. Immunoregulatory prof i les in liver transplant recipients on different immunosuppressive agents. Hum Immunol 2009;70:146-150.
104 Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells. Am J Transplant 2008;8: 1529-1536.
105 Meloni F, Morosini M, Solari N, Bini F, Vitulo P, Arbustini E, et al. Peripheral CD4+ CD25+ Treg cell expansion in lung transplant recipients is not affected by calcineurin inhibitors. Int Immunopharmacol 2006;6:2002-2010.
106 Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 2005;105:4743-4748.
107 Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, Wang Y, et al. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J Immunol 2006; 177:944-949.
108 Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med 2005;201:259-266.
109 Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol 2008;180:5794-5798.
110 Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One 2009;4:e5994.
111 Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol 2004;4:24-34.
112 Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, et al. Cutting edge: Rapamycin augments pathogen-specif i c but not graft-reactive CD8+ T cell responses. J Immunol 2010;185:2004-2008.
113 Knechtle SJ, Pascual J, Bloom DD, Torrealba JR, Jankowska-Gan E, Burlingham WJ, et al. Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. Am J Transplant 2009;9: 1087-1098.
114 De Serres SA, Sayegh MH, Najaf i an N. Immunosuppressive drugs and Tregs: a critical evaluation! Clin J Am Soc Nephrol 2009;4:1661-1669.
115 Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol 2001;167:1945-1953.
116 Demirkiran A, Sewgobind VD, van der Weijde J, Kok A, Baan CC, Kwekkeboom J, et al. Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+FOXP3+ regulatory T cells. Transplantation 2009; 87:1062-1068.
117 Lim DG, Joe IY, Park YH, Chang SH, Wee YM, Han DJ, et al. Effect of immunosuppressants on the expansion and function of naturally occurring regulatory T cells. Transpl Immunol 2007;18:94-100.
118 Sawicka E, Dubois G, Jarai G, Edwards M, Thomas M, Nicholls A, et al. The sphingosine 1-phosphate receptor agonist FTY720 differentially affects the sequestration of CD4+/CD25+ T-regulatory cells and enhances their functional activity. J Immunol 2005;175:7973-7980.
119 Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004;5:987-995.
120 Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol 2004;172:6065-6073.
121 Goldstein DR. Inf l ammation and transplantation tolerance. Semin Immunopathol 2011;33:111-115.
122 Ahmed EB, Daniels M, Alegre ML, Chong AS. Bacterial infections, alloimmunity, and transplantation tolerance. Transplant Rev (Orlando) 2011;25:27-35.
123 Zhai Y, Shen XD, O'Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inf l ammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol 2004;173:7115-7119.
124 Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant 2005;5:1793-1800.
125 Hanidziar D, Koulmanda M, Strom TB. Creating transplant tolerance by taming adverse intragraft innate immunity. F1000 Biol Rep 2010;2:83.
126 Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science 2003;299:1033-1036.
127 Hanidziar D, Koulmanda M. Towards cytoprotection in the peritransplant period. Semin Immunol 2011;23:209-213.
128 Penninga L, Wettergren A, Chan AW, Steinbrüchel DA, Gluud C. Calcineurin inhibitor minimisation versus continuation of calcineurin inhibitor treatment for liver transplant recipients. Cochrane Database Syst Rev 2012;3:CD008852.
Received November 13, 2012
Accepted after revision December 15, 2012
AuthorAff i liations:Center for Organ Transplantation and Department of Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200025, China (Liu XQ, Pei YF and Tao R); Department of Surgery, Central Hospital of Minghang District, Shanghai 201100, China (Hu ZQ)
Ran Tao, MD, Center for Organ Transplantation and Department of Surgery, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, 7W Surgical Building, 197 2nd Ruijin Road, Shanghai 200025, China (Email: taohdac9@yahoo.com)
© 2013, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(13)60002-8
 Hepatobiliary & Pancreatic Diseases International2013年1期
Hepatobiliary & Pancreatic Diseases International2013年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Melanoma in the ampulla of Vater
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Salvage living-donor liver transplantation to previously hepatectomized hepatocellular carcinoma patients: is it a reasonable strategy?
- Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies
- Outcomes of side-to-side conversion hepaticojejunostomy for biliary anastomotic stricture after right-liver living donor liver transplantation
- The pre-Kasai procedure in living donor liver transplantation for children with biliary atresia
