A Two-step Biotechnological Process for Improving Nutrition Value of Feather Meal by Bacillus licheniformis S6
Guo Gang ,Chu Jie,Wang Jun-gaoHe Qiu-xia,and Liu Ke-chun
1 College of Food & Bioengineering,Shandong Institute of Light Industry,Jinan 250353,China
2 Biological Research Institute of Shandong Academy of Science,Jinan 250014,China
3 Zhumadian Huazhong Chia Tai Co.,Ltd.,Zhumadian 463700,Henan,China
Introduction
More than 90% of feather proteins are keratin making poultry feather poorly digested by pepsin,trypsin and papain (Zhang et al.,2009),furthermore,feather protein is deficient in some essential amino acids,such as tryptophan,histidine,lysine and methionine (Moran et al.,1966),both of which are the main limitations to feather utilization.Though the digesti-bility coefficient can reach about 80% by elevating temperature and pressure in vitro,some essential amino acids which are sensitive to heat are destroyed (Gessesse et al.,2003).Considering the thermoenergetic cost of steamtreatment processing of feather and its limitation in improving feather nutrition,investigation into alternative technology with prospects for nutritional improvement,friendly to environment,biological process seems justifiable (Onifade et al.,1998).
Feather meal can be hydrolyzed to release amino acids and peptides in gastrointestinal tract.Some oligopeptides are absorbed intactly into mucosal cells by peptide transporters on the membrane of the intestinal mucosal (Cajori,1933;Hiroshi et al.,1964).Compared with the absorption of free amino acids,oligopeptides have the advantages with lower energy consumption,more rapid absorption rate,less saturation of transporters,no competitive inhibition and maintaining a good amino acids balance after absorption (Matthews et al.,1965;Hiroshi et al.,1984;Webb.Jr.1990).Besides,oligopeptides have positive effects on absorption of free amino acids and protein synthesis (Boza et al.,1995).Enhancing the contents of oligopeptides in feather meal not only improve nutrition value,but also solve the imbalance of some essential amino acids in certain degree.
In our previous work,an aerobic strain of Bacillus licheniformis S6 was isolated which degraded poultry feather and hydrolyze starch (Guo et al.,2012).The objective of the present work was to evaluate the nutrition value of the feather meals treated by solid fermentation at 37℃ using the spores of B.licheniformis S6 and further enzymolysis of the fermentation products utilizing the enzymes produced in solid fermentation at 50℃.
Materials and Methods
Strain and media
Bacillus licheniformis S6 strain was used in this study for feather degrading.Chicken feathers were washed extensively and dried at 75℃ until constant weight,then milled and passed through a 3 mm mesh and stored in sealed bags until use.This material was designated as initial feather meal (IFM).For the seed culture,B.licheniformis S6 grew for 14 h at 37℃ and 120 r • min-1in 100 mL Erlenmeyer flasks containing 30 mL fluid LB medium or grew for 48 h at 37℃ on LB agar slant and was stored at 5℃ until use.The solid fermentations were carried out in 100 mL Erlenmeyer flasks containing 10 g IFM and various volumes of tap water at 37℃ with constant agitation (120 r • min-1) (except ISFM).The media of solid fermentation were previously sterilized by autoclaving at 121℃ for 20 min.The enzymolysis steps were executed by adding sterile water of various volumes at various temperatures according to the experiment design with constant agitation (120 r • min-1) (except ISFM) for 24 h of solid fermentations.
Development of the biotechnological process
Firstly,approximately 1×108spores or cells of B.licheniformis were transferred to 100 mL Erlenmeyer flasks containing 10 g IFM and 10 mL tap water,the pH of tap water was adjusted to 7.0.The samples were taken at intervals for 24 h,pH values (not shown) and soluble protein contents were measured.Secondly,the comparative studies for measuring optimum initial moisture contents (40%,50%,60% and 70% (w/w)),inoculum sizes (1×106,1×107,1×108and 1×109spores) and initial pH values (6.5,7.0,7.5 and 8.0) of tap water were conducted by single factor test.Samples were taken at 96 h after spores inoculating in the media,and soluble protein contents were measured.Thirdly,IFM degradation by B.licheniformis S6 was performed.Samples were taken at intervals for 12 h,and contents of soluble protein and keratinase activity were monitored.Fourthly,the enzymolysis step was conducted after the termination of the solid fermentation at 96 h.The flasks were sealed by PARAFILM® M sealing film,and then the comparative studies of the volumes of added sterile water (pH was adjusted to 8.5 before sterilization) (5,10,15,20 mL) and temperatures (37℃,40℃,45℃,and 50℃) on the contents of oligopeptides and soluble proteins in the following steps were carried out.pH values,soluble protein and oligopeptide contents were estimated.In the evaluation of each step,conditions were chosen that provided maximal soluble protein and oligopeptide contents.
Application of the biotechnological processes
Following the optimization,SFFM (feather meal by solid fermentation),SFEFM (feather meal by solid fermentation and enzymolysis at constant agitation),and ISFM (feather meal by solid fermentation and enzymolysis at intermittent shake) were obtained.
Comparative studies among IFM,SFFM,SFEFM,ISFM and CFM (commercial feather meal,from a local poultry processing plant)
The analyses were carried out to obtain the contents of total nitrogen,crude protein,in vitro digestibility coefficient,contents of soluble proteins and oligopeptides in digestive juice,respectively.The amino acid compositions of CFM,SFEFM and ISFM were estimated.
Treatments and measure methods of samples
The samples in the optimized process were added in 5 mL distilled water (pH was adjusted to 7.0) per gram and mechanically stirred for 2 min on a shaker.An aliquot of the mixture was used to measure pH (not shown).Another mixture was centrifuged (5 000 g for 10 min) and supernatants were served to determine the contents of soluble protein (Wang et al.,2004) and oligopeptide content (the fractions of low molecular weight<2 ku) (Lee et al.,1999;Wang et al.,2004) and keratinase activity (Veselá1 and Friedrich,2009).In the process of comparative studies among IFM,SFFM,SFEFM,ISFM and CFM,the measurements of contents of soluble protein and oligopeptide were the same as above except no distill water adding in digestive juice.pH was measured by using an electronic probe (Sartorius PB-10 pH meter).The total nitrogen and crude protein were assayed as described in AOAC (1990).The in vitro digestibility coefficient was determined by pepsin-pancreatin digestion procedure according to NY/T 915-2004 (2005).The amino acid compositions were assayed according to Veselá1 and Friedrich (2009).
Statistics
All analyses were performed in triplicate.The data obtained was expressed as means or means+SD,analyzed using ANOVA.Differences between means at 5% level (P<0.05) were considered significant.
Results
Optimum of the biotechnological process
There was no significant difference (P>0.05) in inoculating spores or cells of B.licheniformis S6 in solid cultures when the maximum of soluble protein content reached at 96 h (cells versus spores,2 736.46+234.5 μg • mL-1versus 2 560+184.09 μg • mL-1).The maximum soluble protein content (2 738.63+205.40 μg • mL-1) was obtained when the initial moisture content was 60% (w/w) (pH 7.0,1×108spores) (Fig.1a).A fit pH value has beneficial effects on the growth and metabolism of B.licheniformis S6 and keratinase activity.The optimum pH value (7.5) (initial moisture content 60% (w/w),1×108spores) was obtained where the maximum of soluble protein was 2 938.75+398.24 μg • mL-1(Fig.1b).The contents of soluble protein were 2986.53+214.62 μg • mL-1,3 014.29+309.45 μg • mL-1,respectively,when 1×108or 1×109spores were inoculated (Fig.1c).Because the soluble protein concentration was just raised slightly when more than 1×108spores inoculated,the inoculum size of 1×108spores was used in following experiments.According to our previous experiments,the optimization was performed.The maximums keratinase activity (220.03+11.3 U • mL-1) and soluble protein content (2 979.01+148.23 μg • mL-1) reached at 84 h and 96 h,respectively (Fig.2).The content of soluble protein decreased quickly after 96 h.However,the keratinase activity still maintained at high level after 84 h,which indicated that IFM could be degraded further and the nutrition of fermentation product was improved afterwards.Fit volumes of sterile water added and fit temperature had advantages for enzymolysis of feather proteins.The maximum contents of soluble protein and oligopeptide reached to 47 122.54+1 487.30 μg • g-1and 15 104.86+987 μg • g-1at 50℃ (10 mL sterile water),respectively (Fig.3).The optimum volume of added sterile water was 15 mL where the maximum contents of soluble protein and small peptide were 48 967.50+1 296.32 μg • g-1and 17 312.32+896.43 μg • g-1,respectively (Fig.4).
Considering that pH had been at fit value (approximately 8.3) for IFM enzymolysis after 96 h through the self-regulation of B.licheniformis S6 to the environment (8.5 and 50℃ was the optimum pH and temperature of keratinase produced by B.licheniformis S6,respecitively),the influence of sterile water pH on further hydrolyzed IFM was not discussed.

Fig.1 IFM degradation in different initial moisture contents or initial pH values of tap water or inoculum size
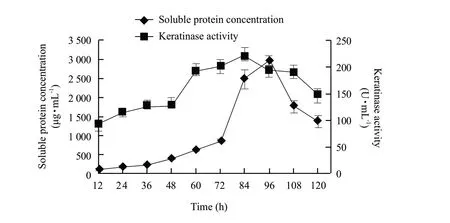
Fig.2 Time courses of soluble protein concentration and keratinase production by B.licheniformis S6 developed in solid cultures
Comparative studies among IFM,SFFM,SFEFM,and CFM
Compared with CFM and IFM,the contents of crude protein of SFFM,SFEFM,and ISFM were lower significantly (p<0.05).The reduction might be results of metabolism of Bacillus licheniformis S6,a thick smell of NH3 was released after 36 h during the fermentation process.
The feather meal was upgraded through the solid fermentation with B.licheniformis S6 and enzymatic further hydrolyzation (Table 1).SFEFM and ISFM were more digestible and released more soluble protein and oligopeptides than IFM.The in vitro digestibility,soluble protein and oligopeptides released in digestive juice of CFM were equivalent statistically (P>0.05) to those of SFFM and lower than those of SFEFM (P<0.05).Though in vitro digestibility and soluble protein released in digestive juice of ISFM were equivalent statistically (P>0.05) to those of CFM,more oligopeptides were released than CFM (P<0.05).The enhancement of oligopeptides might be due to further enzymolysis (mainly keratinase) and the feather protein was degraded into smaller building blocks (Veselá1 and Friedrich,2009),which maybe one of the main reasons that daily weight gains of 3-week-old broiler were higher than commercial feather meal by adding keratinase (Hiroshi et al.,1984).
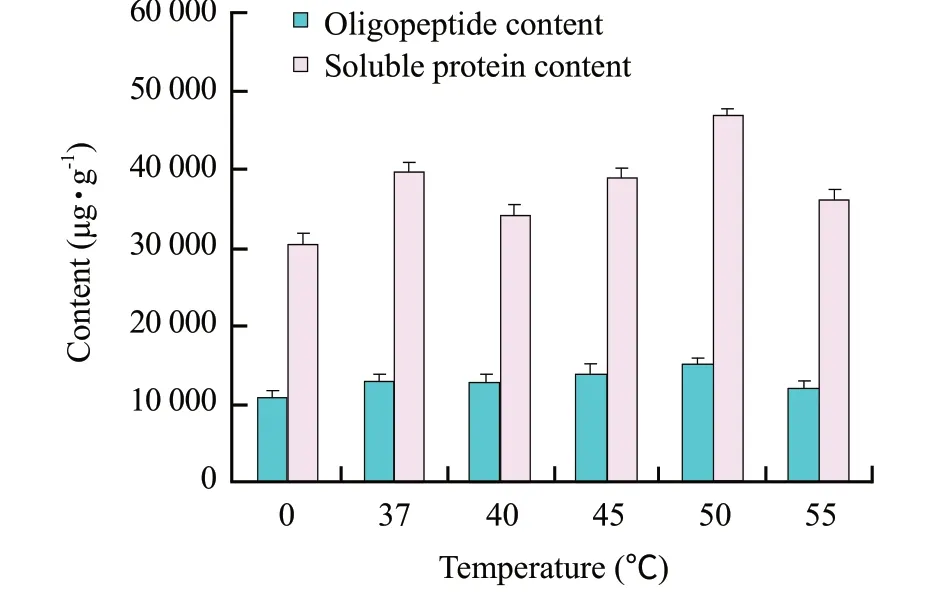
Fig.3 Further hydrolyzed IFM at various temperatures at enzymolysis step
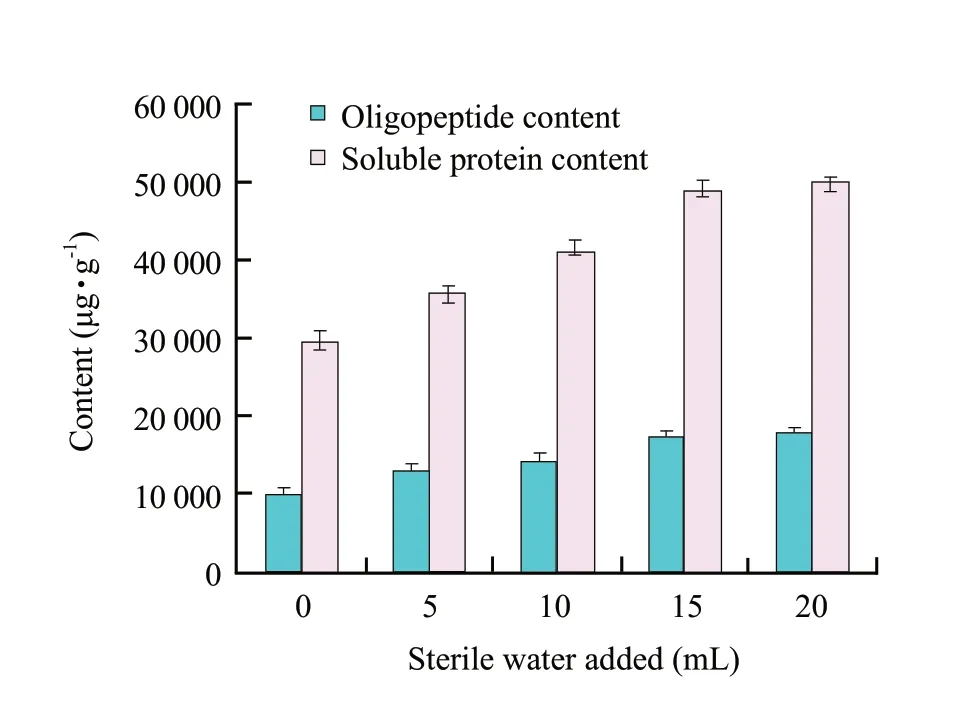
Fig.4 Further hydrolyzed IFM at various contents sterile water added at enzymolysis step

Table1 Quality analysis of IFM,SFFM,SFEFM,ISFM,and CFM
Although the total contents of amino acids in SFEFM were equivalent statistically (P>0.05) to those of CFM,some deficiencies of amino acids in feather protein were higher,such as methionine (0.57% versus 0.35%) histidine (0.65% versus 0.56%) and lysine (2.70% versus 2.66%) (Fig.5),which may be due to the microbial biomass and (or) amino acid anabolism (Veselá1 and Friedrich,2009).
Notably,though the contents of deficiency amino acids in feather protein were not enhanced except lysine (2.70% versus 2.66%),in vitro digestibility of ISFM was equivalent statistically (P>0.05) to CFM and the oligopeptides released were higher than CFM (P<0.05) (Fig.5).
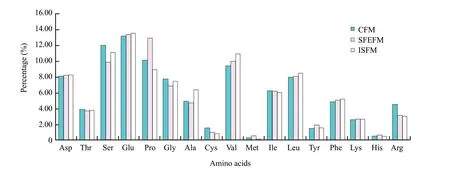
Fig.5 Comparison of amino acid compositions among CFM,SFEFM,and ISFM
Discussion
A two-step method was developed for improving the nutritional value of feather meal by biotechnological process.Degradation of IFM by B.licheniformis S6 in solid fermentation was the first step,which was stopped after 96 h when the maximum content of soluble protein achieved and the keratinase activity still maintained at high level.The cultures obtained from first step (wet weight,16.37+2.24 g) were added in 15 mL sterile water and the finishes were sealed by parafilm.IFM was hydrolysed further by the enzymes produced in first step at 50℃.By the biotechnological process,in vitro digestibility coefficient,contents of soluble protein and oligopeptide in digestive juice of SFEFM were higher than those of SFFM,also the content of oligopeptides in digestive juice of ISFM was higher than those of SFFM,though in vitro digestibility coefficient,content of soluble protein in digestive juice were equivalent statistically (P>0.05) to SFFM.As protein feedstuff,high in vitro digestibility coefficient is necessary for feather meal.In vitro digestibility coefficient of SFFM (79.31%) was higher than the feather meal obtained from solid fermentation using Rhizopus oligosporus (Belewu et al.,2008) and Vibrio sp.strain kr2 (Grazziotin et al.,2006).The result revealed that B.licheniformis S6 was a good strain for improving the digestibility of feather proteins.By the established biotechnological process,the excellent characteristic of B.licheniformis S6 was exerted in improving the feather meal's nutrition value.In vitro digestibility coefficient of SFEFM and ISFM increased to 85.98% and 79.89%,respectively,which were greater than 75% (more than 75% required for animal feed) (Bertsch and Coello,2005).
Some literatures (Brandsen et al.,2001;Moran et al.,1966) reported that the performances of animals would be inhibited when too much CFM were added in diets,which was set by the amino acid imbalance in feather protein (Bertsch and Coello,2005) and some amino acids,such as lysine,histidine and aspartate,were in a low level of absorption in animal gastrointestinal tract (Bielorai et al.,1983).The deficient amino acids,such as methionine,lysine,histidine (Moran et al.,1966),were enhanced and the oligopeptide contents in digestive juice increased up to 63.30 mg • g-1in SFEFM,which indicated that SFEFM had advantages in impairing the deficiency of some nutritionally essential amino acids in feather meal (Hiroshi et al.,1984) and increased the amount added in a diet.
By inoculating spores and cutting off the period of seed cultivation,the fermentation cycle was shortened and fermentation process was simplified.Considering that the only distinction was the way of agitation between SFEFM and ISFM during the complete degradation procedure,the main reason might be that the metabolism and multiplication of B.licheniformis S6 was inhibited partially and the amount of keratinase accumulated decreased in solid fermentation (Onifade et al.,1998).Therefore,the nutrition value of ISFM could be further enhanced by increasing the shaking times every 12 h or (and) inoculum size.Since the quantity and activity of keratinase has played key roles in IFM degradation,the nutrition value of ISFM could be improved by mutagenesis or (and) gene recombination.
Association of Official Analytical Chemists (AOAC).1990.Official Methods of Analysis.15th ed.Washington.DC.pp.69-88.
Belewu M A,Asafa A R,Ogunleke F O.2008.Processing of feather meal by solid state fermentation.Biotechnology,7(3): 589-591.
Bertsch A,Coello N.2005.A biotechnological process for treatment and recycling poultry feathers as a feed ingredient.Bioresource Technology,96: 1703-1708.
Bielorai R,Harduf Z,Iosif B,et al.1983.Apparent amino acid absorption from feather meal by chicks.British Journal of Nutrition,49: 395-399.
Boza J J,Martinez Augustin O,Baro L,et al.1995.Proteinv.enzymetic protein hydrolysates.Nitrogen utilization in starved rats.British Journal of Nutrition,73(1): 65-71.
Brandsen M P,Carter C G,Nowak B F.2001.Effects of dietary protein source on growth,immune function,blood chemistry and disease of Atlantic salmon (Salmo salar L.) parr.Animal Science,73: 105-113.
Cajori F A.1933.The enzyme activity of dogs'intestinal juice and its relation to intestinal digestion.American Journal of Phisiology,104: 659-668.
Gessesse A,Hatti-Kaul R,Gashe B A,et al.2003.Novel alkaline proteases from alkaliphilic bacteria grown on chicken feather.Enzyme and Microbial Technology,32: 519-524.
Grazziotin A,Pimentel F A,de Jong E V,et al.2006.Nutritional improvement of feather protein by treatment with microbial keratinase.Animal Feed Science and Technology,126: 135-144.
Guo G,Chu J,Wang J G,et al.2012.Screening,identification of a strain with high keratinase production and the primary study of its characterization of fermentation.China Animal Husbandry &Veterinary Medicine,39(3): 125-128.
Hara H,Funabiki R,Iwata M,et al.1984.Portal absorption of small peptides in rats under unrestrained conditions.Journal of Nutrition,114: 1122-1129.
Lee G G,Ferket P R,Shih J C H.1991.Improvement of feather digestibility by bacterial keratinase as feed additive.The Journal of the Federation of American Societies for Experimental Biology,59: 1312.
Matthews D M,Leonard L.1965.Absorption of protein digestion products: a review.Gut,6: 411-426.
Moran E T,Summers J D,Slinger S J.1966.Keratin as a source of protein for the growing chick.I.Amino acid imbalance as the cause for inferior performance of feather meal.Poultry Science,45: 1257-1266.
Onifade A A,Al-Sane N A,Al-Musallam A A,et al.1998.A review: potentials for biotechnological applications of keratindegrading microorganisms and their enzymes for nutritional improvement and others keratins as livestock feed resources.Bioresource Technology,66: 1-11.
The Ministry of Agriculture of the People's Republic of China.2005.Hydrolyzed feather meal for feedstuff.(NYIT 915-2004).Beijing.pp.5-6.
Veselá1 M,Friedrich J.2009.Amino acid and soluble protein cocktail from waste keratin hydrolysed by a fungal keratinase of Paecilomyces marquandii.Biotechnology and Bioprocess Engineering,14: 84-90.
Wang L J,Ma Q G,Ji C.2004.Relationship between low molecular weight peptide releasing patterns and feed quality during in vitro enzymatic hydrolysis.Journal of Agricultural Biotechnology,12(4): 416-421.
Webb Jr K.1990.Intestinal absorption of protein hydrolysis products: a review.Journal of Animal Science,68: 3011-3022.
Zhang B,Jiang D D,Zhou W W,et al.2009.Isolation and characterization of a new Bacillus sp.50-3 with highly alkaline keratinase activity from Calotes versicolor faeces.World Journal of Microbiology and Biotechnology,25: 583-590.
 Journal of Northeast Agricultural University(English Edition)2013年3期
Journal of Northeast Agricultural University(English Edition)2013年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Analysis on Dairy Consumption in Heilongjiang Province
- Back Ground Segmentation of Cucumber Target Based on DSP
- Mutagenesis of Arachidonic Acid-producing Mortierella Isabellina and Analyses of Δ6-desaturase Role by qPCR
