Mutagenesis of Arachidonic Acid-producing Mortierella Isabellina and Analyses of Δ6-desaturase Role by qPCR
Yao Di,Yu Chang-qing, ,Yang Jian,and Li Li-na
1 College of Food,Heilongjiang Bayi Agricultural University,Daqing 163319,Heilongjiang,China
2 Quality Supervision and Inspection Center of Agricultural Processed Products on Agriculture Ministry,Daqing 163319,Heilongjiang,China
Introduction
Arachidonic acid is an essential ω-6 polyunsaturated fatty acid (PUFA) that plays a significant role in maintaining the structure and activation of plasmalemmal phospholipids (Chen et al.,1997;Abbadi et al.,2004).ARA has numerous biological activities involved in the nutrition,and is vital in normal brain function,as well as to prevent and treat hypertension,hyperlipidaemia,and diabetes,among other roles (Brick et al.,2000).ARA must often be sourced from food instead of being synthesized in body (Seki et al.,2005).The traditional sources of ARA are animal liver,blood,fish oil and yolk.However,the yields of ARA accumulated from animal tissue are low.ARA production through fermentation believed to be convenient and efficient has been gradually applied in food industry.In microorganisms,elongation and desaturation of the hydrocarbon chain are the two main reactions in ARA synthetic pathway (Certik et al.,2000).Δ6-,Δ5-and Δ4-desaturases are the predominant desaturases in this reaction (Kajikawa et al.,2004,Nicola et al.,2001),of which Δ6-desaturase performs dehydrogenation reactions in the 6th and 7th carbon atoms of the unsaturated fatty acid on the first step of the pathway,which results in the introduction of double bonds (Hong et al.,2002;Liu et al.,2001).
Mortierella isabellina,generally regarded as safe,has been researched and applied in ARA production.It can produce a lot of greases during growing.The contents of ARA is high in these greases (Yu et al.,2009).But,up to now,the lack of high-yield ARA Mortierella isabellina strains retards the production and application of ARA.Therefore,deriving higher ARA-producing mutated strains of Mortierella isabellina using different mutagenesis methods and relationships on Δ6-desaturase mRNA expression levels and ARA production were studied.Evaluation of Δ6-desaturase might provide a foundation for the use of metabolic engineering to construct biosynthetic strains with high-yield ARA.
Materials and Methods
Strains and vectors
Mortierella isabellina (As3.3410) was purchased from Microbiology Institute of Chinese Academy of Science;E.coli BL-21 was preserved in our laboratory;PMD-18 T vector was purchased from TaKaRa Company (DaLian,China).
Chemicals and media
ARA was purchased from Sigma (St.Louis,Mo.USA).TRIzol protocol was purchased from Invitrogen (Invitrogen,USA) Agar,glucose,peptone,yeast extract,and other chemicals were purchased from Shenggong (Shanghai Chemical Company,China).
Mutagenesis and preservation of strains were performed on Potato Dextrose Agar (PDA) medium.The seed culture medium comprised of (per liter) 60 g glucose,2 g yeast extract,1 g KH2PO4,0.3 g MgSO4,A chemically defined medium was used for ARA production,which contained (per liter) 50 g glucose,2 g yeast extract,2 g citrate sodium,2 g KH2PO4,0.5 g MgSO4in distilled water.Initial pH values of all the media were adjusted to 6.0 prior to sterilization at 121℃ for 20 min.
Cultivations of Mortierella isabellina
Erlenmeyer flasks (500 mL) each containing 50-mL seed medium were inoculated with 5% of the spore suspension and incubated at 28℃ at 180 r • min-1for 72 h in a reciprocal shaker.The susbmerged fermentation for ARA production were carried out in 500-mL flasks containing 50 mL chemically defined medium which were performed for 7 days at 30℃ and 200 r • min-1.
Mutagenesis of Mortierella isabellina
Mortierella isabellina As3.3410 was used as a parental strain.Mutations were introduced using four mutagenesis methods: microwave irradiation,UV mutagenesis,protoplast regeneration mutagenesis and UV mutagenesis breeding of the protoplast (Yu et al.,2009).The optimum mutated condition was determined by detecting lethality of mutant strains.The mutant strains were preliminary screened using acetylsalicylic acid screening medium and then were secondary screened by detecting ARA production.The high-yield ARA mutant strains obtained were subcultured to explore their genetic stability.
Determination of ARA contents
The microbial oil of Mortierella isabellina were extracted.ARA contents were analyzed by GC-MS (Agilent,USA),using an capillary column (30 m×0.32 mm×0.5 μm ),the split ritio was 100 : 1,the column was heated at 80℃ for 1 min and increased at 20℃/min to 200℃ and then at 2℃/min to 220℃ and held for 10 min (Liu et al.,2011).
Determination of desaturases activity
The method of determining desaturases activity was according to Yu et al (2009).
Total RNA extraction,PCR and sequence analysis
Total RNA was extracted from Mortierella isabellina according to TRIzol protocol (Invitrogen).Then RNA was reverse transcribed according to the manufacturer's instructions using Oligo dT (TaKaRa,Dalian,China) (Chung et al.,2001;Liu et al.,2011).PCR Amplifying of Δ6-desaturase gene and 18SrRNA gene fragment was performed using primers designed from the Mortierella isabellina sequences,following primers were used:
D6D-F:5'-TCGGGCCGAGATTCTGAAT-3',D6D-R: '-GCCAACGTGCGTGAGAATAA-3',18S-F: 5'-TTCGGAGAAACACCTACACCAA-3',18S-R: 5'-TTCACATTACGTATCGCATTTCG-3'.These primers were also used for real-time PCR.In addition,5'end marked FAM and 3'end marked TEMRA of specific probes were as the followings:
D6D-P: CAACAAGGTGTATGATGTCCGCGA GTTTG,18S-P: TTCACATTACGTATCGCATT TCG.
PCR reaction for Δ6-desaturase gene was carried out using an initial step at 94℃ for 5 min,followed by 30 cycles of denaturation at 94℃ for 40 s,annealing at 55℃ for 50 s,and extension at 72℃ for 45 s.A final extension was carried out at 72℃ for 5 min.PCR reaction for the 18SrRNA gene was carried out using an initial step at 94℃ for 5 min,followed by 30 cycles of denaturation at 94℃ for 40 s,annealing at 52℃ for 45 s,and extension at 72℃ for 40 s.A final extension was carried out at 72℃ for 5 min.Aliquots of PCR reaction products (5 μL) were mixed with 1 μL bromophenol blue glycerine and fractionated by electrophoresis through a 1.5% agarose gel containing 1 μg • mL-1ethidium bromide in a constant 110-V field.DNA fragment was cloned into a pMD18-T vector.Then the recombined plasmids were transformed into E.coli BL-21 and were verified by enzyme digestion and PCR,and were sequenced by Shanghai Sangon Biotechnology Company (Shanghai,China) (Li et al.,2001).
Real-time PCR of Δ6-desaturase gene
Recombinant plasmids were 1 : 10 diluted and took 1 μL for preparation of a standard curve.Δ6-desaturase and 18SrRNA gene standard curve was constructed with TaqMan real-time PCR (Zhou et al.,2006).Realtime PCR conditions of D6D and 18SrRNA were as the followings: 40 cycles of 94℃ for 3 min;94℃ for 30 s;60℃ for 30 s and 72℃ for 30 s;and 40 cycles of 94℃ for 3 min;94℃ for 30 s;59℃ for 30 s and 72℃ for 30 s,respectively.The real-time PCR system reaction volume was 20 μL.
Total RNA was extracted from 7-day culture of different ARA-producing Mortierella isabellina,and from 3-8 day culture of high yield strains YZ-124.The expression level of Δ6-desaturase gene was then measured relative to 18SrRNA gene.
Statistical analysis
The data were analyzed using program SPSS 10.0.Results were expressed as the mean ±SD.Significant differences at p<0.05 were determined using onefactor ANOVA followed by t test.
Results
ARA contents and genetic stability of mutant strains
Mutations were induced using four methods.Microwave irradiation was achieved for high-yielding strain,W35s1-153,with 700 W and total heating time of 35 s.UV mutagenesis of high-yielding strain,Z80s1-17,was obtained using a UV lamp of 20 W at an irradiation distance of 30 cm for 80 s exposure.The enzyme concentration (cellulose : snailase=1 : 1) of high-yielding strain Y-69 obtained by protoplast mutagenesis was 4%,from a reaction temperature of 30℃,with a hydrolysis time of 7.5 h and protoplast formation rate was 78.4%.UV mutagenesis breeding of the protoplasts of high-yielding strain YZ-124 was obtained using a UV lamp at 20 W,with an irradiation distance of 30 cm and an exposure time of 40 s.After fermentation tests,ARA contents from these highyield strains were determined according to GC-MS (Fig.1).The highest ARA content (4.72 g • L-1) was measured for YZ-124 obtained by UV mutagenesis breeding of the protoplasts;this was an increase of 576% of the starting strain (Table 1).A comparison of the genetic stability for subsequent generations of high-yield strains showed no significant reduction in ARA content (Fig.2).To explore trends in the Mortierella isabellina fermentation process for the production of ARA,ARA contents of the mutant YZ-124 strain were determined over an increasing number of fermentation days.The results showed that ARA content increased with an increase in the age of YZ-124 cells (Table 2).
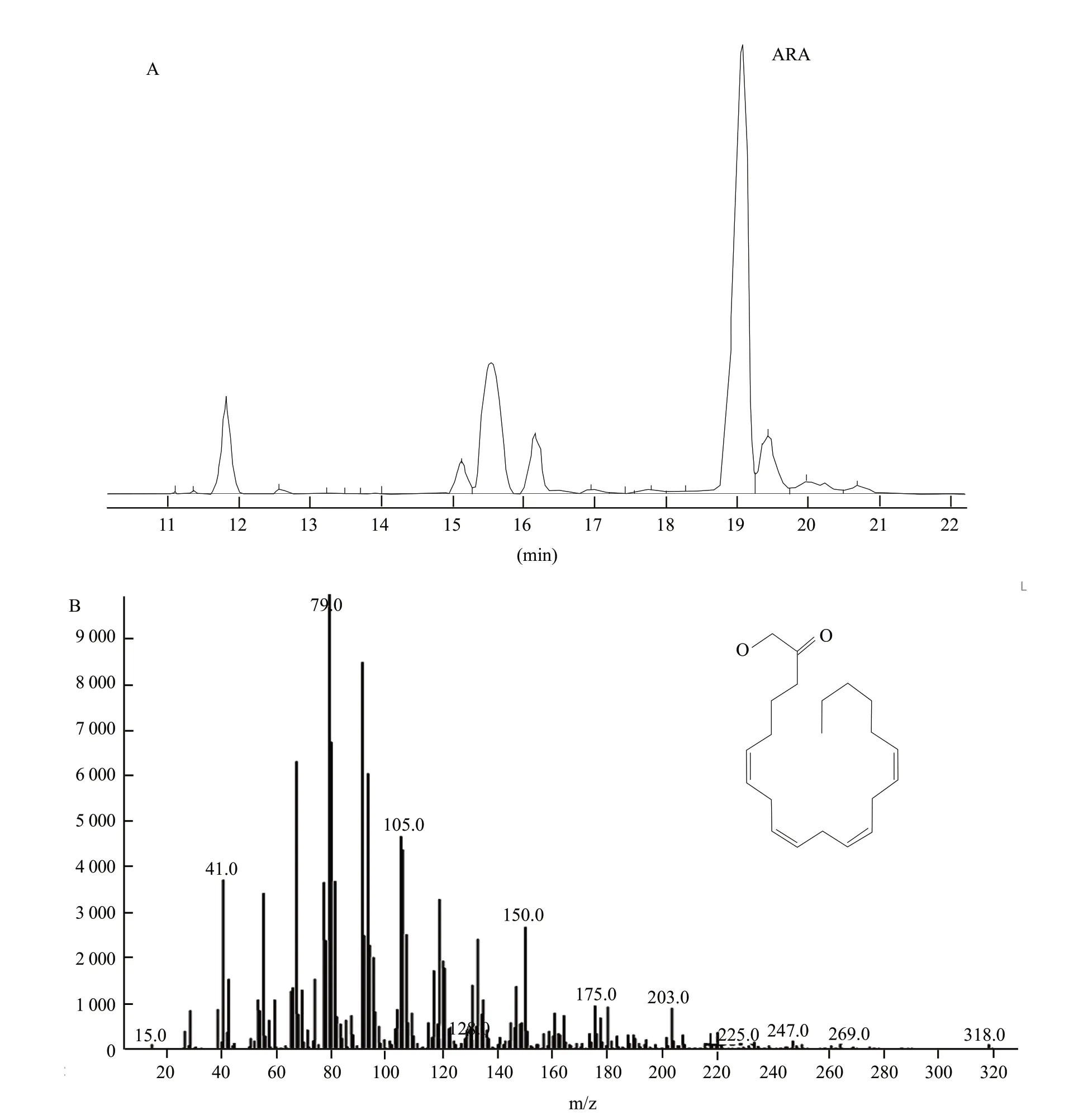
Fig.1 GC-MS analysis of ARA contents from Mortierella isabellina
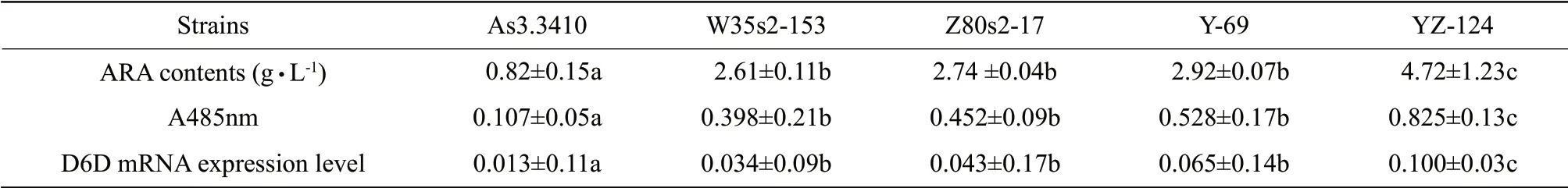
Table1 ARA contents,TTC staining degree and Δ6-desaturase mRNA expression level of five strains Mortierella isabellina in 7 days (mean±SD,n=3)
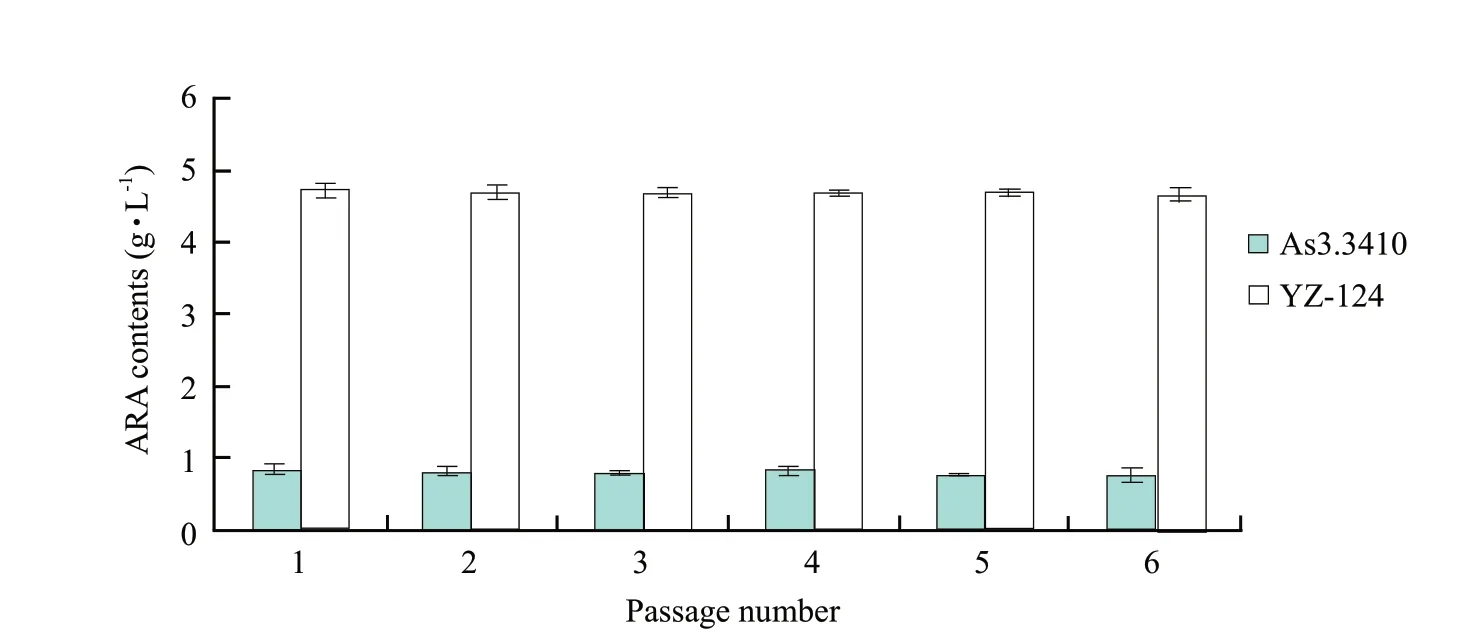
Fig.2 ARA yields of Mortierella isabellina As3.3410 and YZ-124 propagated for six generations

Table2 ARA contents,staining degree and Δ6-desaturase mRNA expression level of Mortierella isabellina YZ-124 in several fermented days (mean±SD,n=3)
Desaturase activity analysis
The degree of TTC staining measures the desaturase activity,with a darker colour indicating a higher activity.The original strain As3.3410 showed minimal activity,with mutant strains showing higher activity levels.YZ-124 strain was among the highest,indicating that the desaturase activity of the highyield ARA mutant strains was higher than that of the original strain (Table 1).The desaturase activity of longer fermentation cultures of Mortierella isabellina YZ-124 was determined.After 3-8 days in culture (Table 2),there was a time-dependent increase in activity,with significant increase observed within the first 6-7 days that became relatively stable after 7 days.
Clone on Δ6-desaturase and 18SrRNA in Mortierella isabellina
Δ6-desaturase and 18SrRNA gene fragments in Mortierella isabellina cultures with varying ARA production were amplified by RT-PCR (Fig.3) and the cloned recombinant plasmids were sequenced.Sequencing results showed 99.8% and 99.6% homology with Δ6-desaturase gene from GenBank (AF306634) and 18SrRNA from GenBank Mortierella sp.(FJ861405.1),respectively,which indicated that Δ6-desaturase gene was highly conserved in Mortierella isabellina.
Δ6-desaturase gene mRNA expression level
A standard curve was drawn with the logarithm of different copy numbers against the cycle number (Ct) of the fluorescent signal.The results of target genes (Fig.4) showed an R2of 0.999 and linear equation y=–3.35x+43.70 for 104-107Δ6-desaturase gene copies in the reaction and an R2of 0.992 and linear equation y=–3.07x+49.42 for 106-101018SrRNA gene copies in the reaction.
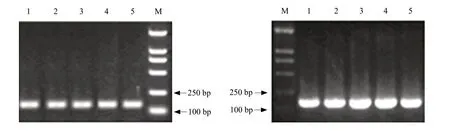
Fig.3 GC-MS analysis of ARA contents from Mortierella isabellina
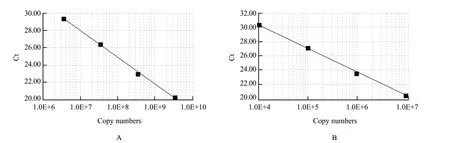
Fig.4 Standard curve of (A) Δ6-desaturase and (B) 18SrRNA
The two standard curves obtained a good linear relationship,in accordance with the requirements of quantitative PCR.Using the standard curve,Δ6-desaturase and 18SrRNA gene mRNA expressions were calculated;the results were shown as Δ6-desaturase mRNA expression levels relative to 18SrRNA gene expression.Δ6-desaturase mRNA expression levels varied among five strains of Mortierella isabellina after 7 days in culture (Table 1).Δ6-desaturase mRNA expression of Mortierella isabellina AS3.3410 was the lowest,with higher expression observed for each of the other four high-yield ARA mutated strains.Overall,YZ-124 showed significantly higher expression of Δ6-desaturase gene as compared with the other four strains (P<0.05).
For the high-yield ARA YZ-124 strain over 3-8 days of fermentation (Table 2),mRNA expression of Δ6-desaturase was the lowest at 3 days in culture,but gradually increased over time.By 6-7 days,relative mRNA expression of Δ6-desaturase was significantly enhanced and remained in an upward trend.
Relation of ARA contents,desaturase activity and Δ6-desaturase mRNA expression
ARA contents,desaturase activity and Δ6-desaturase mRNA expression in Mortierella isabellina AS3.3410,W35s2-153,Z80s2-109,Y-69 and YZ-124 cultured under the same conditions showed significant differences.The results showed that mRNA expression level of Δ6-desaturase correlated with ARA production in cultured oil (Table 1).Increasing levels of Δ6-desaturase gene expression mirrored increasing production of ARA as well as increased desaturation activity.Furthermore,this relationship extended to the high-yielding strain YZ-124,with different ARA productions and syntheses (Table 2).This result indicated that Δ6-desaturase gene expression directly affected ARA biosynthesis and implicated an important role for Δ6-desaturase in Mortierella isabellina ARA synthesis.
Discussion
Mutations were introduced in Mortierella isabellina original strain,AS3.3410,using four mutagenesis methods.While microwave and UV mutagenesis induced some mutant strains,their uses in the longterm were limited,as the thick cell wall of fungal spores would confer resistance.However,protoplasts were generally obtained in the logarithmic phase,meaning that the cells were likely to be more sensitive to their environment and external mutagens.ARAproducing capacity of mutant strains obtained by UV mutagenesis protoplasts was induced by more than a single mutation method in this study,and the stable,genetic,high-yield ARA strain YZ-124 was obtained.Over six generations,there was no significant reduction in ARA production,indicating that the strain would reach industry requirements.However,the genetic variation of the mutation strain was absolute,and its stability was relative.Therefore,active general rejuvenation measures must be taken,and the pure separation and ARA content determination must be conducted before there were some changes in the strain production.
Δ6-desaturase plays an important role in polyunsaturated fatty acids synthesis,and is thus a key enzyme in ARA biosynthesis pathway (Zhang et al.,2007).Δ6-desaturase catalyses a rate-limiting step in ARA biosynthesis (Kaijikawa et al.,2008;Li et al.,2011).Consequently,it is important to understand the effects of Δ6-desaturase in ARA production in Mortierella isabellina (Wang et al.,2007).This study compared ARA production and desaturase activity,as well as mRNA expression level of Δ6-desaturase gene in Mortierella isabellina cultures before and after insult with mutagenesis to examine the relationship between desaturase and ARA contents in oil.A correlation among Δ6-desaturase gene mRNA expression levels,desaturase activity and ARA production was observed,indicating that Δ6-desaturase plays a key role in ARA synthesis in Mortierella isabellina.These results shed light on the regulation of ARA production and screening of high-yield ARA,and lend further support for the potential to synthesize high-yielding ARA species by metabolic engineering technology.
As with other eukaryotes,changes in Δ6-desaturase gene expression may be due to changes in DNA transcription and translation (Huang et al.,1999;Lu et al.,2009;Wan et al.,2009).Δ6-desaturase nucleotide sequence does not change among different ARA-producing strains of Mortierella isabellina,suggesting that differences might exist in gene transcription and translation,rather than changes at DNA level.However,divergence was between Δ6-desaturase mRNA level and the activity of other mutagenesis strains,and the original strain was small.While this suggested that translation regulation of gene expression had few effects,further studies should be required to confirm this.
Abbadi A,Domergue F,Bauer J.2004.Biosynthesis of very long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation.Plant Cell,16: 2734-2748.
Brick E E,Garfield S,Hoffman D R.2000.A randomized controlled trial of early dietary supply of long chain polyunsaturated fatty acids and mental development in term infants.Dev Med Child Neurol,42: 173-181.
Lounds C,Eagles J,Carter A T,et al.2007.Spore germination in Mortierella alpina is associated with a transient depletion of arachidonic acid and induction of fatty acid desaturase gene expression.Arch Microbiol,188: 299-305.
Chung H W.2001.Reverse transcriptase PCR (RT-PCR) and quantitative-competitive PCR (QC-PCR).Exp Mol Med,33: 85-97.
Yu C Q,Li L N.2009.Breeding of arachidonic acid producing Mortierella isabellina by ultraviolet mutation.Acta Microbiologica Sinica,49: 44-48.
Jiang D H,Ji H,Ye Y,et al.2011.Studies on screening of higher γ-aminobutyric acid-producing Monascus and optimization of fermentative parameters.Eur Food Res Technol,232: 541-547.
Eiji Sakuradani,Yuriko Hirano,Nozomu Kamada.2004.Improvement of arachidonic acid production by mutants with lower n-3 desaturation activity derived from Mortierella alpine IS-4.Appl Microbiol Biotechnol,66: 242-248.
Huang Y S,Chaudhary S,Thurmond J M.1999.Cloning of Δ12and Δ6-desaturases from Mortierella alpina and recombinant produc-tion of γ-linolenic acid in Saccharomyces cerevisiae.Lipids,34: 649-659.
Hong H,Datla N,Reed DW.2002.High-level production of γ-linolenic acid in Brassica juncea using a Δ6desaturase from Pythium irregulare.Plant Physiol,129: 354-362.
Liu J M,Li D R,Yin Y T,et al.2011.Δ6-desaturases from Mortierella alpine: cDNA cloning,expression,and phylogenetic analysis.Biotechnol Lett,33: 1985-1991.
Kaijikawa M,Matsui K,Ochiai M.2008.Production of arachidonic and eicosapentaenoic acids in plants using bryophyte fatty acid Δ6desaturase,Δ6elongase,and Δ5desaturase genes.Biosci Biotechnol Biochem,72: 435-444.
Kajikawa M,Yamato K T,Kohzu Y.2004.Isolation and characterization of Δ6-desaturase,an ELO-Like enzyme and Δ5-desaturase from the Liverwort Marchantia Polymorpha and production of arachidonic and elcosapentaenoic acids in the methylotrophic yeast Pichia pastoris.Plant Mol Biol,54: 335-352.
Li T,Dauenpen,Meesapyodsuk.2011.Molecular analysis of Δ6 desaturase and Δ6elongase from Conidiobolus obscurus in the bio-synthesis of eicosatetraenoic acid,a ω3 fatty acid with nutraceutical potentials.Appl Microbiol Biotechnol,90: 591-601.
Liu L,Li M C,Hu G W.2001.Identification of Mortierella isabellina M6-22 Δ6-fatty acid desaturase by heterologous expression in Saccharomyces cerevisiae.Acta Microbiologica Sinica,41: 397-401.
Li M C,Liu L,Zhang L.2009.Cloning and sequencing analysis of Δ6-fatty acid desaturase gene from Mortierella isabellina.Mycosystema,20: 44-50.
Lu H.et al.,2009.Identification and characterization of a novel Δ6-fatty acid desaturase gene from Rhizopusnigricans.Molecular Biology Reports,36: 2291-2297.
Meesapyodsuk D,Reed D W,Covello P S.2007.Primary structure,regioselectivity,and evolution of the embrane-bound fatty acid desaturases of Claviceps purpurea.J Biol Chem,282: 20191-20199.
Certik M,Shimizu S.2000.Kinetic analysis of oil biosynthesis by an arachidonic acid-producing fungus,Mortierella alpina 1S-4.Appl Microbiol Biot,54: 224-230.
Nicola H,Morris A,Douglas R T.2001.A vertebrate fatty acid desaturase with Δ5and Δ6activities.Biochemistry,25: 14304-14309.
PfafflM W,Hageleit M.2001.Validities of mRNA quantification using recombinant RNA and recombinant DNA external calibration curves in real-time RT-PCR.Biotechnol Lett,23: 275-282.
Takeno S,Sakuradani E,Tomi A.2005.Trasformation of oil-producting fungus,Mortierella alpine IS-4 using zeocin,and application to arachidonic acid production.J Biosci Bioeng,100: 617-622.
Wang D,Li M,Wei D Y.2007.Identification and functional characterization of the delta 6-fatty acid desaturase gene from Thamnidium elegans.J Eukaryot Microbiol,54: 110-117.
Wan X,Zhang Y B,Wang P.2009.Production of gamma-linolenic acid in Pichia pastoris by expression of a delta-6 desaturase gene from Unninghamella echinulata.J Microbiol Biotechn,19: 1098-1102.
Zhang X W,Li M C,Wei D S.2007.Disruption of the fatty acid Δ6-desaturase gene in the oil-producing fungus Mortierella isabellina by homologous recombination.Curr Microbiol,55: 128-134.
Yu C Q.2009.Preparation and its controlling mechanism of Arachidonic acid produced with Mortierlla isabellina at high-yield.Jilin University,Changchun.
Zhou X R,Robert S,Singh S.2006.Heterologous production of GLA and SDA by expression of an Echium plantagineum Δ6-desaturase gene.Plant Sci,170: 665-673.
 Journal of Northeast Agricultural University(English Edition)2013年3期
Journal of Northeast Agricultural University(English Edition)2013年3期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Analysis on Dairy Consumption in Heilongjiang Province
- Back Ground Segmentation of Cucumber Target Based on DSP
- A Two-step Biotechnological Process for Improving Nutrition Value of Feather Meal by Bacillus licheniformis S6
