Physiological and Biochemical Changes of IrlVHA-c gene Transgenic Tobacco Seedlings and Self-crossed Progeny Under NaHCO3 Stress
Li Shan-shan,Yang Tao,Bi Xiao-lu,Fu Guo-hua,and Wang Jin-gang
College of Horticulture,Northeast Agricultural University,Harbin 150030,China
Introduction
Plants are often subjected to high soil salinity during their life cycle in many areas of the globe with the amplification of the saline-alkali soil area.Selecting fine variety to adapt to alkaline environment to develop and use salt lick is the tool to improve the situation.Therefore,many researchers focused on the research of the saline-alkali tolerant plant.Iris lactea is the moderate alkali resistance plant.Its flowers and fruits as usual even in the PH value of up to 7.9-8.8 (Meng et al.,2003).So it has high research value in northeast of China where there is the large area of alkaline land.
Plant vacuolar ATPase is a hetero-multimeric enzyme composed of 12 different subunits and these subunits assemble into two independent functional complexes,the hydrophilic V1complex and a hydrophobic,membrane integral V0complex (Wricha Tyagi et al.,2006).Subunit c is a part of the V0and it is the first muligenic family reported in eukaryote V-ATPase (Sze et al.,1992).The saltresistance of VHA-c gene in Limonium bicolor (Ma,2008),barley (Dietz et al.,1995),Arabidopsis (Kluge et al.,1999),S.salsa (Li Pinghua et al.,2003),Helianthus annuus,(Rafael,2000),Beta Vulgaris L.(Lehr et al.,1999),tomato (Binzel et al.,1995) has been shown to be up-regulated in salinity stress,but the IrlVHA-c gene was not.At present,a fragment of VHA-c gene in Iris lacteal has been cloned by Fu et al.(2010) through RT-PCR,after RACE,the full length gene had been obtained,and by using degenerate primer PCR and the 5'-RACE technique,Zhou et al.(2010) had cloned five different isoforms (IrlVHA-c1.c5) of V-ATPase subunit c from a Japanese iris cDNA library,and they verified that IrlVHA-c is important for the function of V-ATPase gene in Iris lactea.However,few proofs have verified the functional verification on the alkali stability of the VHA-c,and we have not encountered any reports on whether IrlVHA-c gene has been expressed in transgenic plant offspring.Therefore,this experiment inspected the alkali stability of the IrlVHA-c gene from the point of view of the physiological and biochemical.
Materials and Methods
Materials
The transgenic lines of tobacco seedlings harboring VHA-c in Iris lacteal (T0),and their self-crossed progeny (T1) were provided by the Northeast Agricultural University.The non-transgenic tobacco was taken as the contrast.
Material culture
Leaves of the non-transgenic tobacco,T0and T1transgenic tobacco were rinsing for 2 h and then surface-sterilized with 5% sodium hypochlorite solution in a beaker for 5 min with constant agitation in the clean benches.After the treatment,NaClO was removed.The leaves were dipped in 70% ethanol for 10 s,and then washed at least three times with sterile water.Subsequently,the leaves were incised into 1 cm×1 cm and grown in MS medium contained 0.78 g • L-1ager,30 g • L-1surose,1.0 m g • L-16-BA and 0.1 mg • L-1NAA,and were transferred to fresh medium every 10 days until the caespitose shoots were grown.Seedlings were grown in modified Hoagland nutrient solution for 2 weeks.
NaHCO3 stress
The T0,T1of transgenic tobacco and non-transgenic tobacco which grew consistently with each other were respectively moved into Hoagland nutrient solution containing NaHCO3concentration of 0,100,200,300,and 400 mmol • L-1to NaHCO3stress.After 7 days,the leaves of tobacco seedlings with different treatments on the same location were taken,wrapped with tinfoil,and then frozen in the –80℃ freezer.
Physiological index measurements
SOD,CAT and POD activities were determined using the method of Zou (1995),MDA content,chlorophyll content,soluble sugar content,electrical conductivity and proline content using the method of Chen and Wang (2002),polyphenol oxidase activity using the method of Raymond (1993).
Statistical analysis
Data was analyzed by Microsoft Excel WPS and the statistic program SPSS 17.0.
Results
SOD,POD,and CAT activity assay
The plant could produce enzymes scavenging reactive oxygen species and antioxidant substances under alkali stress,such as SOD,POD and CAT,to protect film structure and reduce the injury of the plants.Especially,SOD was the enzyme which inactivated the O2-.Its activity increased with exposure to alkalinity,and could be indicative of a need to protect against increased O2-.production (Parihar,1997 ).
The SOD,POD and CAT activities of the transgenic tobacco were all notably higher than those of the non-transgenic plants (Figs.1-3).What's more,three antioxidases'activities of the transgentic tobacoo significantly increased in comparison to the control at different elevated concentrations of NaHCO3,but it was not as the same at the concentration of 400 mmol • L-1.In contrast,three antioxidases'activities of the control showed irregular changes,and the plants could not survive at the NaHCO3concentration as high as 400 mmol • L-1.The values in Tables 1-3 meant significant difference at P<0.05 level,and existed extremely significant difference at P<0.01 level as compared with the control,but the CAT activity of T0A and T1A under 200 and 300 mmol • L-1stress were not the same.It showed that the alkali resistance of the tobacoo transformed IrlVHA-c was improved.

Fig.1 CAT activity comparison of strains under NaHCO3 stress at different concentrations

Fig.2 POD activity comparison of strains under NaHCO3 stress at different concentrations

Fig.3 SOD activity comparison of strains under NaHCO3 stress at different concentrations

Table1 Difference analyses of strains under NaHCO3 stress on CAT activity
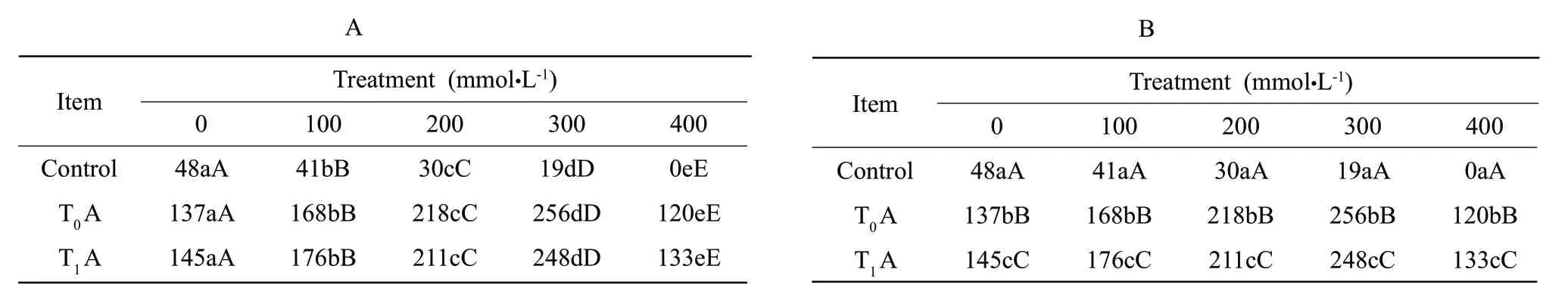
Table2 Difference analyses of strains under NaHCO3 stress on POD activity

Table3 Difference analyses of strains under NaHCO3 stress on SOD activity
MDA content assay
The MDA was the product of lipid peroxidation.Some studies had reported that the MDA could indirectly respond to the membrane damage status (Gong,1989).Fig.4 clearly showed a comparison of the MDA content in transgentic tobacoo with NaHCO3concentration increasing,from which we could find no signif icant change except a sharp raise in its value at the concentration of 400 mmol • L-1.On the contrary,the content of non-transgentic tobacoo increased significantly and the value could not be determined at 400 mmol • L-1NaHCO3.Moreover,the MDA content in non-transgentic tobacoo was visibly higher than that in transgenic tobacco.The values in Table 4 showed that the strains under 100 mmol • L-1NaHCO3and normal conditions had no significant difference at P<0.05 and P<0.01 levels.Therefore,the alkali resistance of the transgentic tobacoo was higher.

Fig.4 MDA content comparison of strains under NaHCO3 stress at different concentrations

Table4 Difference analyses of strains under NaHCO3 stress on MDA content
Relative electrical conductivity assay
Fig.5 showed that 100-300 mmol • L-1NaHCO3had no significant change on the relative electrical conductivity of transgentic tobacoo in respect to that of the control.But it increased significantly when the NaHCO3concentration further increased.Contrarily the relative electrical conductivity of non-transgentic tobacoo increased significantly up to 300 mmol • L-1NaHCO3,and no value was obtained at 400 mmol • L-1.The values in Table 5 meant significant difference at P<0.05 level and existed extremely significant difference at P<0.01 level as compared with the control.It is indicated that the alkali resistance of the tobacoo transformed IrlVHA-c was notably improved.
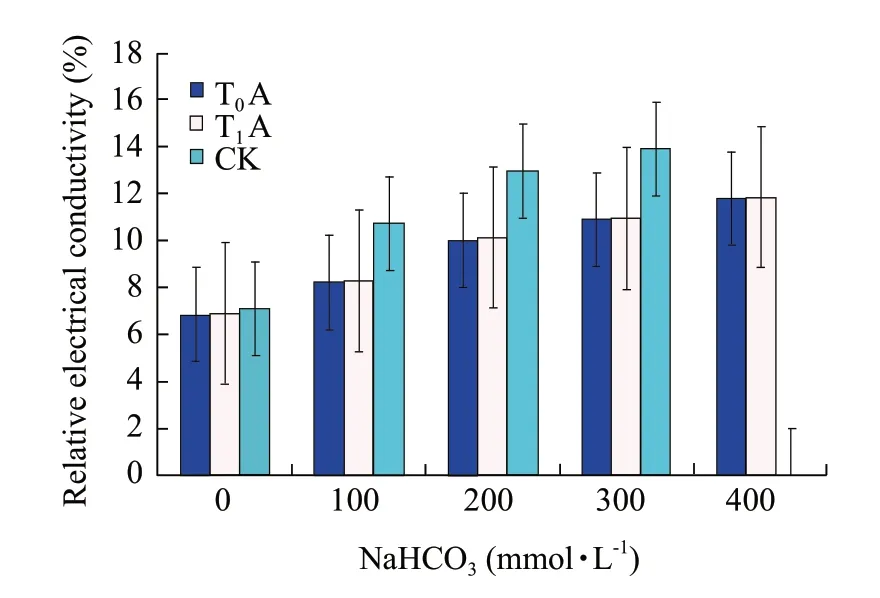
Fig.5 Relative electrical conductivity comparison of NaHCO3 stress at different concentrations
Chlorophyll content assay
Salinity induced lower biomass accumulation and chlorophyll content accordance with the previous findings (Gadallah,1996;Gadallah and Ramdan,1997).The experiment derived the similar conclusion.
From Fig.6 we can see that NaHCO3decreased the chlorophyll content in respect to that of the control.The tendency was observed in all cultivars,but with different degrees.In contrast with transgentic tobacoo,NaHCO3at 100 mmol • L-1and higher reduced the chlorophyll content of leaves in non-transgenic tobacco more significantly.The content of transgentic tobacoo was approximately from 1.43 to 3.33 fold more than that of non-transgenic tobacco.Compared with the control,values in each column in Table 6 showed significant difference at P<0.05 level and extremely significant difference at P<0.01 level.In short,the alkali resistance of the tobacoo transformed IrlVHA-c was notably improved.

Fig.6 Chlorophyll content comparison of strains under NaHCO3 stress at different concentrations

Table5 Difference analyses of strains under NaHCO3 stress on relative electrical conductivity

Table6 Difference analyses of strains under NaHCO3 stress on chlorophyll content
Soluble sugar content assay
It can be seen from Fig.7 that soluble sugar content in seedlings of transgentic tobacoo increased signif icantly up to 200 mmol • L-1NaHCO3,but at higher alkalinity decreased to values lower than that of control.But soluble sugar contents in T0and T1transgentic tobacoo were maintained more or less the same.In contrast,the content in seedlings of non-transgentic tobacoo decreased significantly,and at 200 mmol • L-1NaHCO3it was slightly lower than that of the 100 mmol • L-1NaHCO3,Moreover,non-transgentic tobacoo could not survive when the concentration was up to 400 mmol • L-1.Compared with the control,the values in Table 7 meant significant difference at P<0.05 level and extremely significant difference at P<0.01 level.We could draw the conclusion that the transgentic tobacoo was more alkali-resistant.

Fig.7 Soluble sugar content comparison of strains under NaHCO3 stress at different concentrations

Table7 Difference analyses of strains under NaHCO3 stress on soluble sugar content
Proline content assay
Fig.8 indicated that proline content in seedlings of transgentic tobacoo increased significantly up to 200 mmol • L-1NaHCO3,but increased slightly up to 300 mmol • L-1,and at higher alkalinity decreased prominently.NaHCO3did not induce any conspicuous changes between T0and T1transgentic tobacoo.But no noticeable changes in non-transgenic tobacco were seen.The content in non-transgentic tobacoo was considerably lower than that in transgentic tobacoo.And the crest value was presented when the concentration of NaHCO3was as high as 100 mmol • L-1.The values in Table 8 showed that the strains under 100 and 400 mmol • L-1NaHCO3indicated no significant difference at P<0.05 and P<0.01 level.So the non-transgenic tobacco was less alkali-resistant.

Fig.8 Proline content comparison of strains under NaHCO3 stress at different concentrations
Polyphenol oxidase activity assay
As it is shown in Fig.9,NaHCO3at 100 mmol • L-1and higher significantly increased polyphenol oxidase activity,but NaHCO3at 300 mmol • L-1and more reduced slightly.T0and T1transgenic tobacco had not significant difference.Contrary to that of nontransgentic tobacoo seedlings,maximum increase in polyphenol oxidase activity was achieved at 100 mmol • L-1,and then the activity reduced,which indicated that the transgenic tobacco was more alkali-resistant.The values in Table 9 meant significant difference at P<0.05 level and extremely significant difference at P<0.01 level,except three strains under normal conditions.

Fig.9 Polyphenol oxidase activity comparison of strains under NaHCO3 stress at different concentrations
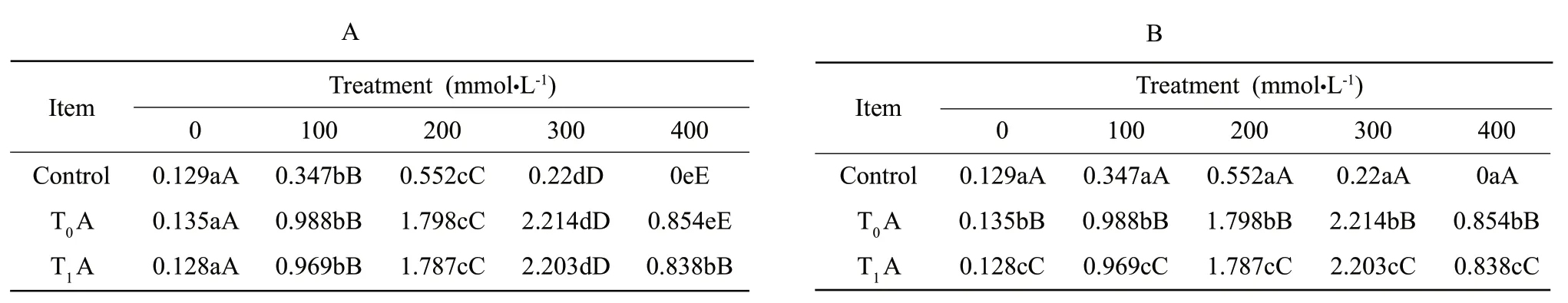
Table8 Difference analyses of strains under NaHCO3 stress on proline content
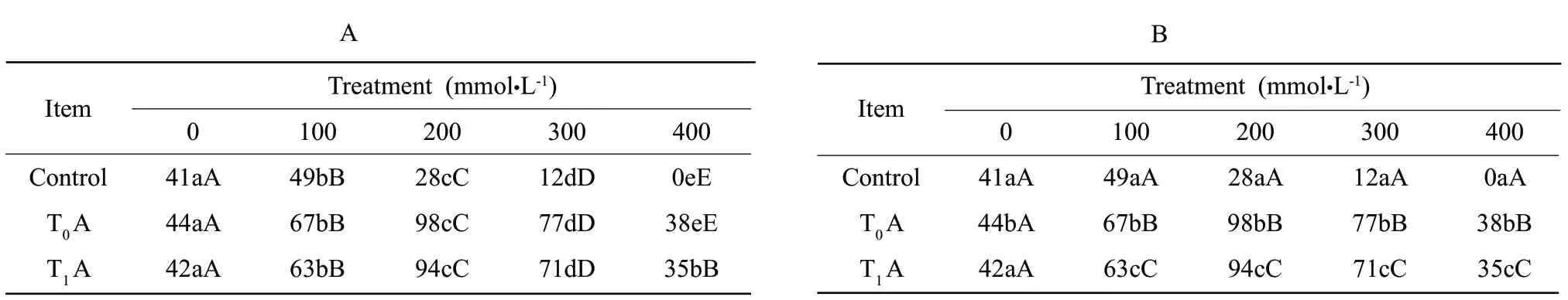
Table9 Difference analyses of strains under NaHCO3 stress on polyphenol oxidase activity
Discussion
The saline-alkali stress included osmotic stress,ionic stress secondary stress and the stress generated by these two (Zhu,2001).Under the normal condition,the formatting and scavenging reaction of free radicals kept dynamic equilibrium in plants.However,when the plant was in the adversity condition of salinealkali stress,this equilibrium would be broken,and the plants would be injured.According to Stewart and Lee (1974),proline was osmotic agent.Other researches had suggested that proline was a source of energy,carbon and nitrogen for the recovering tissues (Blum and Ebercon,1976).In the present study,on one hand,the IrlVHA-c gene could enhance the content of soluble sugar,free proline and other osmolytes to maintain the osmotic balance between the protoplast and the environment and structural integrity.On the other hand,by means of increasing in active oxygen scavenging enzyme activity of SOD and POD,scavenging free radicals and reducing the accumulation of reactive oxygen species,the IrlVHA-c gene could reduce tobacco in leave membranous under NaHCO3stress formation of oxidation products of MDA.Proline accumulation was a symptom of damage (Hanson,1977).So the alkali resistance of the tobacco transformed IrlVHA-c was noticeably improved.
In order to verify whether the IrlVHA-c gene improved stress tolerance in transgenic plants,five concentrations of NaHCO3were used to evaluate the tolerance of transgenic plants by the determination of the physiological indicators.The results of one-way anova showed that significant difference at P<0.05 level,and existed extremely significant difference at P<0.01 level as compared with the control on the determination of POD,soluble sugar content,chlorophyll content,relative electrical conductivity,which indicated these indicators were more relatively to alkali-resistance.
Conclusions
The results indicated that physiological properties of both the transgenic tobacco and the non-transgenic tobacco were subject to vary degrees of the impacts.However,transgenic tobacco lines damaged significantly less than non-transgenic lines.Transgenic lines of tobacco maintained a high activity up to 200 mmol • L-1NaHCO3,and the activity was slightly lower at 300 mmol • L-1NaHCO3.When the concentration of NaHCO3was as high as 400 mmol • L-1the seedlings were badly hurt.While the non-transgenic lines of tobacco could maintain viable up to 100 mmol • L-1NaHCO3,and they could not survive at 400 mmol • L-1NaHCO3.Therefore,the alkali resistance of the tobacoo transformed IrlVHA-c was noticeably improved.In addition,the activities of T0and T1transgentic tobacoo were maintained more or less the same,which meant the IrlVHA-c gene was inherited in the self-progeny of the transgentic tobacoo.
Binzel M L.1995.NaCl-indiced accumulation of tonoplast and plasmamembrane H+-ATPase message in tomato.Physiologia Plantarum,94: 722-728
Blum A,Ebercon A.1976.Genotypic responses in sorghum to drought stress.III.Free proline accumulation and drought resistance.Crop Sci,16: 428-431.
Chen J X,Wang X F.2002.Plant physiology experiments guidance.South China University of Technology Press,Guangdong.pp.120-121.
Dietz K J,Rudloff S,Ageorges A,et al.1995.SuE of the vacuolar H+-ATPase of Hordeum vulgare L.: cDNA cloning,expression and immunological analysis.The Plant Journal,8: 521-529.
Fu H J,Zhou A M,Che D D,et al.2010.Cloning and sequence analysis of vacuolar-type H+-ATPase subunit c family from Iris lactea.Crops Magazine,3: 60-64.
Gadallah M A A.1996.Abscisic acid,temperature and salinity interactions on growth and some mineral elements in Carthamus plants.Plant Growth Regul,20: 225-236.
Gadallah M A A,Ramdan T C.1997.Effects of zinc and salinity on growth and anatomical structure of Carthamus tinctorius L.Biol Plant,39: 411-418.
Gong M,Ding N C,He Z Y,et al.1989.Correlation between lipid preoxidation damage and ultrastructural changes of mesophyll cells in barley and wheat seedlings.Acta Botanica Sinica,31(11): 841-846.
Hanson A D,Nelson C E,Eversen E H.1977.Evolution of free proline accumulation as an index of drought resistance using two contrast-ing barley cultivars.Crop Sci,17: 720-726.
Ma H.2008.Expression of Genes from Limonium bicolor under NaHCO3Stress and Functional Identification of the VHA-c.Journal of Northeast Forestry University,Harbin.
Meng L,Zhang G F,Zhao M L.2003.The excellent manner plants in water protected slope: Iris lactea.New Agricultural Technology,3: 38-39.
Parihar M S,Javeri T,Hemnani T,et al.1997.Responses of superoxide dismutase,glutathione peroxidase and reduced glutathione antioxidant defenses in gills of the freshwater catfish (Heteropneustes fossilis) to short-term elevated temperature.J Therm Biol,22(2): 151-156.
Kirsch M,Viereck R,et al.1999.cDNA and genomic cloning of xugar beet V-type H+-ATPase subunit A and c isoforms: evidence forcoordinate induction in response to high salinity.Plant Molecular Biology,39: 463-475.
Kluge C,Golldack D,Dietz K J.1999.Subunit D of the vacuolar H+-ATPase of Arabidopsis thaliana.Biochim Biophys Acta,1419: 105-110.
Li P H,Chen M,Wang B S.2002.Effect of K+nutrition on growth and activity of leaf tonoplast V-H+-ATPase and V-H+-PPase of Suaeda salsa under NaCl stress.Journal of Integrative Plant Biology,44(4): 433-440.
Ratajczak R.2000.Structure,function and regulation of the plant vacuolar H+-translocating ATPase.Biochimica et Biophysica Acta,1465: 17-36.
Raymond J,Pakariyathan N,Azanza J L.1993.Purification and some properties of polyphenoloxidases from sunflowers seeds.Phytochemistry,34: 927-931.
Stewart G R,Lee J A.1974.The role of proline accumulation in halophytes.Planta,120: 279-289.
Sze H,Ward J M,Lai S.1992.Vacuolar-H+-ATPases from plants.Bioenerg Biomembr,24: 371-381.
Tyagi W,Singla-Pareek S,Nair S,et al.2006.A novel isoform of ATPase c subunit from pearl millet that is differentially regulated in response to salinity and calcium.Plant Cell Rep,25: 156-163.
Zhou A M,Wu D,Che D D,et al.2011.Cloning of vacuolar H+-ATPase subunit c genes from Japanese iris,and functional characterization in yeast.Journal of Forestry Research,22(3): 361-366.
Zhu J K.2001.Plant salt toleranee.Trends plant Sci,6: 66-72.
Zou Q.1995.Plant Physiology and Biochemistry experimental guidance.China Agriculture Press,Beijing.pp.96-99.
 Journal of Northeast Agricultural University(English Edition)2013年1期
Journal of Northeast Agricultural University(English Edition)2013年1期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Journal of Northeast Agricultural University (English Edition)
- Advanced Progress on Adaptive Stress Response of Oenococcus oeni
