Simultaneous determination of telmisartan and amlodipine in human plasma by LC-MS/MS and its application in a human pharmacokinetic study
Vasu Bau Ravi, Jaswanth Kumar Inamadugu, Nageswara Rao Pilli,Vudagandla Sreenivasulu, Venkateswarlu Ponneri
aResearch Studies, Rayalaseema University, Kurnool 518002, India
bWellquest Clinical Research Laboratories, Ramanthapur, Hyderabad 500013, India
cBioPolymer and Thermophysical Lab, Department of Chemistry, Sri Venkateswara University, Tirupati 517502, India
dAnalytical and Environmental Chemistry Division, Department of Chemistry, Sri Venkateswara University, Tirupati 517502, India
1. Introduction
Telmisartan (Fig.1) is a highly selective angiotensin II type 1 receptor antagonist,widely used in the treatment of hypertension and heart failure [1-3]. It can selectively block the angiotensin type 1 receptor without affecting other receptor systems involved in cardiovascular regulation. Amlodipine (Fig.1), a dihydropyridine calcium antagonist,is prescribed for the treatment of angina and hypertension.Amlodipine is a peripheral arterial vasodilator that acts directly on vascular smooth muscle to cause a reduction in peripheral vascular resistance and blood pressure [4-6].

Figure 1 Chemical structures of telmisartan (A),amlodipine (B),and carbamazepine (C).
A combination of antihypertensive agents can better control blood pressure and reduce the number and severity of side effects than a monotherapy.Both angiotensin II type 1 receptor blockers and calcium channel blockers were shown to be efficacious in reducing cardiovascular risk. Telmisartan and amlodipine fixed dose combinations have been demonstrated in numerous clinical trials to be highly effective in lowering blood pressure and suggest that the combined use might be more effective in treating hypertension than a monotherapy [7,8]. Twynsta®, a new single pill combination therapy of telmisartan and amlodipine, is approved by US FDA for the treatment of hypertension.
As per the literature, several LC-MS/MS methods have been reported for the determination of telmisartan [9-16] and amlodipine[17-27]individually or with other drugs in biological samples.To date, no LC-MS/MS method has been reported for the simultaneous determination of telmisartan and amlodipine in human plasma. For pharmacokinetic and bioequivalence studies of telmisartan associated with amlodipine, it is recommended to perform the quantitation of telmisartan and amlodipine simultaneously.The present work describes a simple,selective and sensitive method, which employs a simple solid-phase extraction technique for sample preparation and liquid chromatography with electrospray ionization-tandem mass spectrometry for simultaneous quantitation of telmisartan and amlodipine in human plasma.The application of this assay method to a clinical pharmacokinetic study in healthy male volunteers following oral single pill administration of telmisartan and amlodipine is described.
2. Experimental
2.1. Chemicals and reagents
The reference samples of telmisartan (99.89%) and amlodipine(99.59%) were purchased from Neucon Pharma Ltd. (Goa,India). Carbamazepine (99.27%) used as an internal standard(IS) in this study, was obtained from Hetero Labs Ltd.(Hyderabad, India). Water used for the LC-MS/MS analysis was prepared from Milli Q water purification system procured from Millipore (Bangalore, India). Acetonitrile and methanol were of HPLC grade and purchased from J.T. Baker (Phillipsburg,USA).Analytical grade ammonium acetate and acetic acid were purchased from Merck (Mumbai, India). Oasis®HLB 1 cm3(30 mg) extraction cartridge was purchased from Waters Corporation (Milford, Massachuselts, USA). The control K2-EDTA human plasma sample was procured from Doctor's Pathological Laboratory (Hyderabad, India).
2.2. Instrumentation and chromatographic conditions
An HPLC system (Shimadzu, Kyoto, Japan) consisting of a Hypurity advance C18column (50 mm×4.6 mm, 5 μm;Thermo Electron Corporation, Bellefonte, PA), a binary LC-20AD prominence pump, an autosampler (SIL-HTc) and a solvent degasser (DGU-20A3) was used for the study. Aliquots of the processed samples (20 μL) were injected into the column, which was kept at 30°C. The isocratic mobile phase,a mixture of acetonitrile and 5 mM ammonium acetate (pH-4.0) (50:50, v/v) was delivered at 0.8 mL/min into the electrospray ionization chamber of the mass spectrometer. Quantitation was achieved with MS-MS detection in positive ion mode for both the analytes and the internal standard using a MDS Sciex API-4000 mass spectrometer (Foster City, CA, USA)equipped with a TurboionsprayTMinterface at 500°C.The ion spray voltage was set at 5500 V. The source parameters, viz.the nebulizer gas, curtain gas, auxiliary gas and collision gas were set at 45, 20, 45 and 10 psi, respectively. The compound parameters viz. the declustering potential (DP), collision energy (CE), entrance potential (EP) and collision cell exit potential (CXP) were 80, 65, 10, 15 V for telmisartan, 50, 15,10, 12 V for amlodipine and 75, 27, 10, 5 V for the IS.Detection of the ions was carried out in the multiple-reaction monitoring mode (MRM), by monitoring the transition pairs of m/z 515.2 precursor ion to the m/z 276.2 for telmisartan,m/z 409.3 precursor ion to the m/z 238.2 for amlodipine and m/z 237.2 precursor ion to the m/z 194.1 product ion for the IS. Quadrupoles Q1 and Q3 were set on unit resolution. The analysis data obtained were processed by Analyst softwareTM(version 1.4.2).
2.3. Preparation of standard solutions
Primary stock solutions of telmisartan and amlodipine for preparation of standard calibration curve and quality control(QC) samples were prepared from separate weighing. The stock solutions of telmisartan (1000 μg/mL) and amlodipine(100 μg/mL)were prepared in methanol and these stocks were stored at 2-8°C; they were found to be stable for 10 days.From these stock solutions, appropriate dilutions were made using a mixture of acetonitrile and water (50:50, v/v) as a diluent, to produce working standard solutions of telmisartan and amlodipine.The primary stock solution of carbamazepine(1000 μg/mL) was prepared in methanol. A working concentration of the internal standard (500 ng/mL) solution was prepared in the diluent (acetonitrile and water, 50:50, v/v).
2.4. Preparation of calibration curve standards and quality control samples
Calibration samples were prepared by spiking 950 μL of control human plasma with the appropriate working standard solution of the each analyte (25 μL dilution of telmisartan and 25 μL of amlodipine). Calibration curve standards consisting of a set of ten non-zero concentrations ranging from 2.01 to 400.06 ng/mL for telmisartan and 0.05 to 10.01 ng/mL for amlodipine were prepared. Samples for the determination of precision and accuracy were prepared by spiking control human plasma in bulk with telmisartan and amlodipine at appropriate concentrations and 350 μL plasma aliquots were distributed into different tubes. The QC samples prepared for each analyte are: for telmisartan—2.05 lower limit of quantification,(LLOQ), 6.02 (LQC), 200.79 (MQC) and 340.32 ng/mL(HQC); and amlodipine—0.05 (LLOQ), 0.15 (LQC), 5.04(MQC) and 8.54 ng/mL (HQC). All the samples were stored at -70±5°C for subsequent use.
2.5. Sample processing
A 250 μL aliquot of human plasma sample was mixed with 25 μL of the internal standard working solution(500 ng/mL of carbamazepine). To this, 250 μL of Milli Q water was added after vortex mixing for 10 s. The sample mixture was loaded onto a Oasis®HLB 1 cm3(30 mg) extraction cartridge that was pre-conditioned with 1.0 mL of methanol followed by 1.0 mL water. The extraction cartridge was washed with 1.0 mL of water. Telmisartan, amlodipine and carbamazepine were eluted with 0.5 mL of mobile phase. Aliquot of 20 μL of the extract was injected into the LC-MS/MS system.
2.6. Method validation
A thorough validation of the method was carried out as per the US FDA guidelines [28]. The method was validated for selectivity,sensitivity,matrix effect,linearity,precision,accuracy,recovery,dilution integrity and stability.Selectivity of the method was assessed by analyzing six blank human plasma matrix samples.The responses of the interfering substances or background noises at the retention time of the telmisartan and amlodipine are acceptable if they are less than 20%of the response of the lowest standard curve point or LLOQ. The responses of the interfering substances or background noise at the retention time of the internal standard are acceptable if they are less than 5% of the response of the working internal standard.
Sensitivity was established from the background noise or response from six spiked LLOQ samples. The six replicates should have a precision of ≤20% and an accuracy of ±20%.Matrix effect is investigated to ensure that precision,selectivity and sensitivity are not compromised by the matrix. Matrix effect was checked with six different lots of K2-EDTA plasma.Three replicate samples each of LQC and HQC were prepared from different lots of plasma (36 QC samples in total).
Linearity was tested for telmisartan and amlodipine in the concentration range of 2.01-400.06 and 0.05-10.01 ng/mL, respectively. For the determination of linearity, standard calibration curves containing at least 10 points (non-zero standards) were plotted and checked. In addition, blank plasma samples were also analyzed to confirm the absence of direct interferences, but these data were not used to construct the calibration curve. The acceptance limit of accuracy for each of the back-calculated concentrations is ±15% except LLOQ, where it is ±20%. For a calibration run to be accepted at least 67% of the standards, the LLOQ and upper limit of quantification (ULOQ) are required to meet the acceptance criterion, otherwise the calibration curve was rejected. Five replicate analyses were performed on each calibration standard. The samples were run in the order from low to high concentration.
Intra-day assay precision and accuracy were determined by analyzing six replicates at five different QC levels in the same day on two runs.Inter-day assay precision and accuracy were determined by analyzing six replicates at four different QC levels on five different runs. The acceptance criteria included accuracy within ±15% deviation (SD) from the nominal values, except LLOQ QC, where it should be ±20% and a precision of ≤15% relative standard deviation (RSD), except for LLOQ QC, where it should be≤20%. Whereas batch acceptance criteria included 67% for over all quality control samples and 50% at each level respectively.
Recovery of the analytes from the extraction procedure was determined by comparing the peak areas of the analytes in spiked plasma samples (six each of low, middle, and high QC samples) with those of the analytes in samples prepared by spiking the extracted drug-free plasma samples with the same amounts of the analytes at the step immediately prior to chromatography.Similarly,recovery of the IS was determined by comparing the mean peak areas of the extracted QC samples (n=6) with those of the IS in samples prepared by spiking the extracted drug-free plasma samples with the same amounts of the IS (500 ng/mL) at the step immediately prior to chromatography.
The dilution integrity exercise is performed with an aim to validate the dilution test to be carried out on higher analyte concentrations above the ULOQ during real time analysis of subject samples. Dilution integrity experiment was carried out at 1.6 times the ULOQ concentration for both the analytes.Six replicates each of 1/2 and 1/4th concentrations were prepared and their concentrations were calculated by applying the dilution factors 2 and 4.
Stability tests were conducted to evaluate the analyte stability in stock solutions and in plasma samples under different conditions. The stock solution stability at room temperature and refrigerated conditions (2-8°C) was performed by comparing the area response of the analytes(stability samples) with the response of the sample prepared from fresh stock solution.Bench top stability(10 h),processed samples stability (autosampler stability for 48 h, wet extract stability for 40 h and reinjection stability for 24 h), freezethaw stability (four cycles), and long-term stability (30 days)were performed at LQC and HQC levels using six replicates at each level.Samples were considered to be stable if assay values were within the acceptable limits of accuracy(±15%SD)and precision (≤15% RSD).
2.7. Pharmacokinetic study design
A pharmacokinetic study was performed in healthy male subjects (n=6). The ethics committee approved the protocol and the volunteers provided with informed written consent.Blood samples (1 mL) were collected following oral administration of 80/10 mg fixed dose combination tablet of telmisartan and amlodipine at pre-dose and 0.25, 0.5, 0.75, 1, 1.25,1.5, 2, 3, 4, 5, 6, 7, 7.5, 8, 8.5, 9, 10, 12, 16, 24, 36, 48, 72 and 96 h, in K2-EDTA vacutainer collection tubes (BD, Franklin,NJ,USA). The tubes were centrifuged at 3200 rpm for 10 min at 4°C and the plasma was collected. Immediately after collection, the plasma samples were stored at -70°C till their use. Plasma samples were spiked with the IS and processed as per the extraction procedure described earlier. Along with the clinical samples, the QC samples at low, middle and high concentration levels were assayed in triplicate and were distributed among the unknown samples in the analytical run; not more than 33% of the QC samples were greater than±15% of the nominal concentration. Plasma concentrationtime profile of each analyte was analyzed by non-compartmental method using WinNonlin Version 5.1.
3. Results
3.1. Mass spectrometry
Mass parameters were tuned in both positive and negative ionization modes for the analytes. Good response was achieved in positive ionization mode. Data from the MRM mode were considered to obtain better selectivity. Protonated form of each analyte and IS[M+H]+ion was the parent ion in the Q1spectrum and was used as the precursor ion to obtain Q3product ion spectra.The most sensitive mass transition was monitored from m/z 515.2 to 276.2 for telmisartan, m/z 409.3 to 238.2 for amlodipine and from m/z 237.2 to 194.1 for the IS. As earlier publications have discussed the details of fragmentation patterns of telmisartan [9], amlodipine [17] and the IS [29], we are not presenting the data pertaining to this.
3.2. Method development
The chromatographic conditions, especially the composition of mobile phase, were optimized through several trials to achieve good resolution and symmetric peak shapes for the analytes as well as a short run time.Separation was attempted using various combinations of acetonitrile and buffer with varying contents of each component on different columns like C8and C18of different makes like Chromolith, Hypersil, Hypurity advance, Zorbax,Kromasil and Intertsil.It was found that a mixture of acetonitrile and 5 mM ammonium acetate(pH-4.0)(50:50,v/v)could achieve this purpose and was finally adopted as the mobile phase.Hypurity advance C18(50 mm×4.6 mm, 5 μm) gave a good peak shape and response even at LLOQ level for both the analytes and the IS.The retention time of telmisartan,amlodipine and the IS was low enough (1.52, 0.65 and 1.23 min) allowing a small run time of 2.5 min.
A simple solid-phase extraction (SPE) technique was employed for the sample preparation in this work and provides high recoveries of the drugs. A good internal standard must mimic the analyte during extraction and compensate for any analyte on the column. Isotope-labeled analyte was not available to serve as the IS,so,in the initial stages of this work, several compounds were investigated to find a suitable IS and finally carbamazepine was found to be best for the present purpose. Carbamazepine was evaluated for precision and accuracy and extraction recovery of the internal standard was good and reproducible.
3.3. Selectivity and chromatography
The degree of interference by endogenous plasma constituents with the analytes and the IS was assessed by inspection of chromatograms derived from processed blank plasma sample. As shown in Figs. 2 and 3 no significant direct interference in the blank plasma traces was observed from endogenous substances in drug-free plasma at the retention time of the analytes and the IS.
3.4. Sensitivity
The lowest limit of reliable quantification for the analytes was set at the concentration of the LLOQ. The precision and accuracy at LLOQ concentration were found to be 4.35% and 101.89% for telmisartan, 7.59% and 103.35%for amlodipine.
3.5. Matrix effect
No significant matrix effect was observed in all the eight batches of human plasma for the analytes at LQC and HQC concentrations. The precision and accuracy for telmisartan at LQC concentration were found to be 1.66%and 100.30%and at HQC level they were 0.37% and 99.94%, respectively.Similarly, the precision and accuracy for amlodipine at LQC concentration were found to be 1.69% and 102.52%, and at HQC level they were 1.12% and 100.12%, respectively.
3.6. Linearity
Ten-point calibration curve was found to be linear over the concentration range of 2.01-400.06 ng/mL for telmisartan and 0.05-10.01 ng/mL for amlodipine. After comparing the two weighting models (1/x and 1/x2), a regression equation with a weighting factor of 1/x2of the drug to the IS concentration was found to produce the best fit for the concentrationdetector response relationship for both the analytes in human plasma. The mean correlation coefficient of the weighted calibration curves generated during the validation was 0.99.
3.7. Precision and accuracy
Accuracy and precision data for intra- and inter-day plasma samples for telmisartan and amlodipine are presented in Table 1. The assay values on both the occasions (intra- and inter-day)were found to be within the accepted variable limits.
3.8. Extraction efficiency
A simple solid-phase extraction with Oasis®HLB 1 cm3(30 mg) extraction cartridge was proved to be robust and provided cleanest samples. The recoveries of analytes and the IS were good and reproducible. The mean overall recoveries(with the precision range) of telmisartan, amlodipine and the IS were 87.16±0.50% (1.53-3.80%), 82.31±0.61% (2.08-4.50%) and 78.33±0.85% (2.13-2.36%), respectively.

Figure 2 Typical MRM chromatograms of telmisartan (left panel) and the IS (right panel) in human blank plasma (A), and human plasma spiked with IS (B), a LLOQ sample along with IS (C).
3.9. Dilution integrity
The upper concentration limits can be extended to 640.09 ng/mL for telmisartan and 16.02 ng/mL for amlodipine by 1/2 and 1/4 dilutions with screened human blank plasma. The mean back-calculated concentrations for 1/2 and 1/4 dilution samples were within 85-115% of their nominal value. The coefficients of variation (%CV) for 1/2 and 1/4 dilution samples were less than 15%.
3.10. Stability studies
In the different stability experiments carried out, viz. bench top stability (10 h), autosampler stability (48 h), freeze-thaw stability (4 cycles), reinjection stability (24 h), wet extract stability (40 h at 2-8°C) and long-term stability at -70°C for 30 days, the mean % nominal values of the analytes were found to be within ±15% of the predicted concentrations for the analytes at their LQC and HQC levels(Table 2).Thus,the results were found to be within the acceptable limits during the entire validation.
Stock solutions of telmisartan, amlodipine and the IS were found to be stable for 10 days at 2-8°C. The percentage stability (with the precision range) of telmisartan, amlodipine and the IS was 103.02% (1.20-2.21%), 99.44% (1.30-1.63%)and 102.27% (2.29-2.58%), respectively.
3.11. Pharmacokinetic study results
In order to verify the sensitivity and selectivity of this method in a real-time situation, the present method was used to test for telmisartan and amlodipine in human plasma samples collected from healthy male volunteers (n=6). The mean plasma concentration vs time profile of telmisartan (presented up to 24 h in order to depict the plot with clarity) and amlodipine is shown in Fig.4(A) and (B), respectively and the pharmacokinetic data are listed in Table 3. These values were in close proximity when compared with earlier reported values [14,21].
4. Discussion
To date,no reports are available for the simultaneous quantification of telmisartan and amlodipine in any of the matrices.Validated methods are essential for the determination of telmisartan and amlodipine concentrations in human plasma for bioequivalence studies. This is, to the best of our knowledge,the first validation report for an LC-MS/MS method for the simultaneous assay of telmisartan and amlodipine using the simple solid-phase extraction procedure. The reported method is simple, rugged and rapid due to utilization of short run time of 2.5 min for each sample analysis.
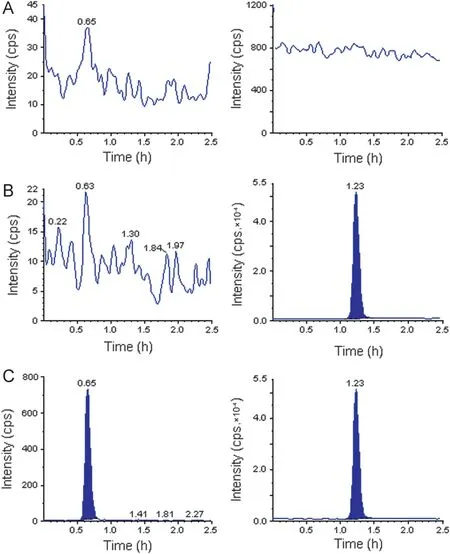
Figure 3 Typical MRM chromatograms of amlodipine (left panel) and the IS (right panel) in human blank plasma (A), and human plasma spiked with IS (B), a LLOQ sample along with IS (C).
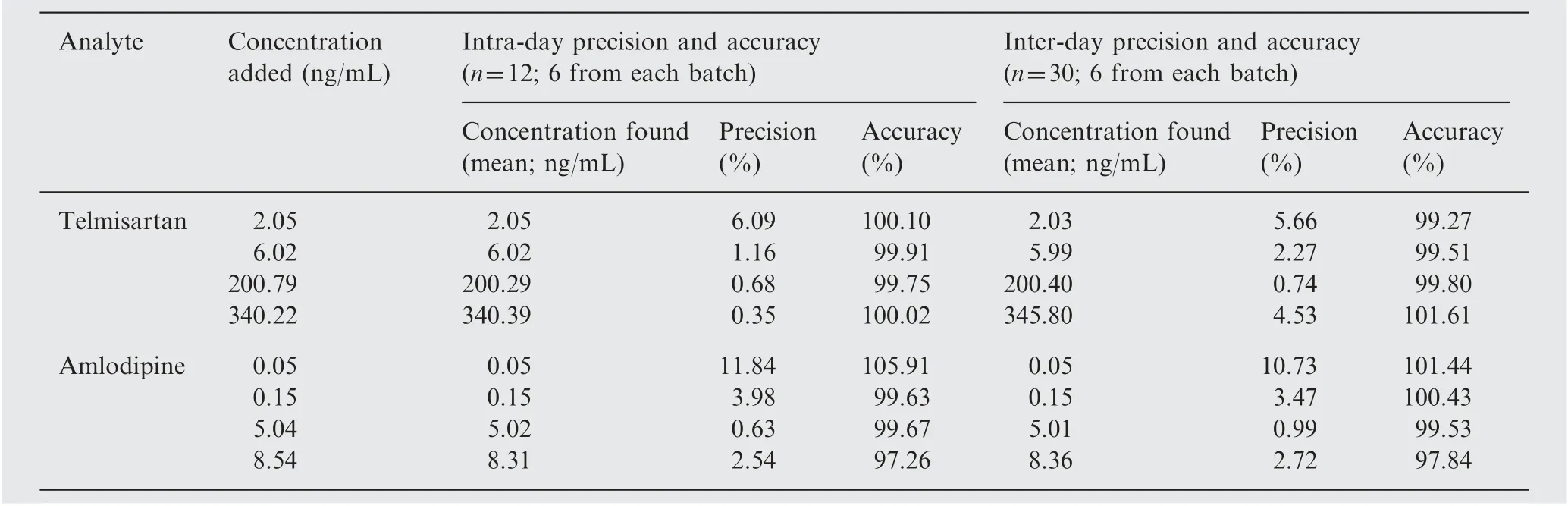
Table 1 Precision and accuracy of the method for determining telmisartan and amlodipine in plasma samples.

Table 2 Stability samples result for telmisartan and amlodipine in human plasma (n=6).

Figure 4 Mean plasma concentration-time profile of telmisartan(A), and amlodipine (B), in human plasma following the oral administration of 80/10 mg fixed dose combination of telmisartan and amlodipine tablet to healthy volunteers (n=6).
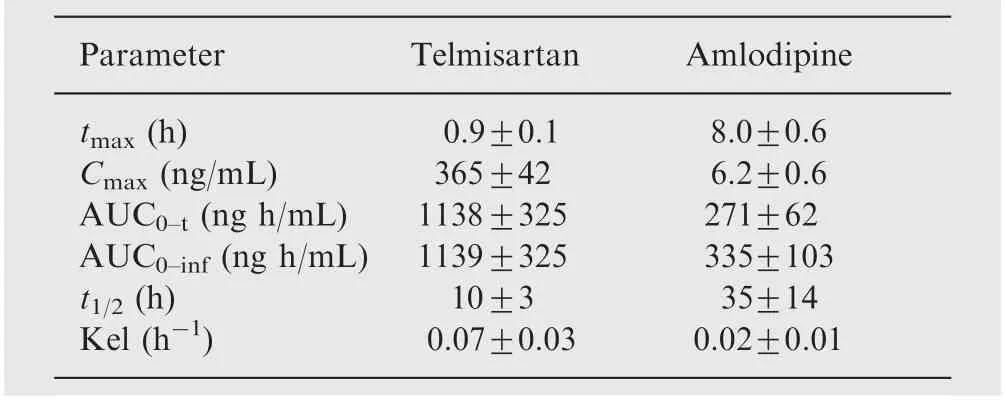
Table 3 Pharmacokinetic parameters of telmisartan and amlodipine (n=6, Mean±SD).
5. Conclusions
The LC-MS/MS assay reported in this paper is rapid, simple,specific and sensitive for simultaneous quantification of telmisartan and amlodipine in human plasma and is fully validated according to commonly acceptable FDA guidelines.The method showed suitability for pharmacokinetic studies in humans. The cost-effectiveness,simplicity of the assay and usage of solid phase extraction, and sample turnover rate of less than 2.5 min per sample, make it an attractive procedure in high-throughput bioanalysis of telmisartan and amlodipine. From the results of all the validation parameters,we can conclude that the developed method can be useful for BA/BE studies and routine therapeutic drug monitoring with the desired precision and accuracy.
The authors gratefully acknowledge Wellquest Clinical Research Laboratory, Hyderabad for providing necessary facilities for carrying out this study.
[1] R.W. Ruth, J.C. William, D.I. John, et al., Nonpeptide angiotensin II receptor antagonists: the next generation in antihypertensive therapy, J. Med. Chem. 39 (1996) 625-656.
[2] B. Pitt, M.A. Konstam, Overview of angiotensin II-receptor antagonists, Am. J. Cardiol. 82 (1998) 47S-49S.
[3] S. Yusuf, K.K. Teo, J. Pogue, et al., Telmisartan, ramipril, or both in patients at high risk for vascular events,N.Engl.J.Med.358 (2008) 1547-1559.
[4] DR. Abernethy, Pharmacokinetics and pharmacodynamics of amlodipine, Cardiology 80 (1992) 31-36.
[5] G. Kungys, H. Naujoks, C. Wanner, Pharmacokinetics of amlodipine in hypertensive patients undergoing haemodialysis,Eur. J. Clin. Pharmacol. 59 (2003) 291-295.
[6] J.L.Reid,P.A.Meredith,R.Donnelly,et al.,Pharmacokinetics of calcium antagonists, J. Cardiovasc. Pharmacol. 12 (1988) 22-26.
[7] M.D. Moen, Telmisartan/amlodipine single-pill combination in hypertension, Am. J. Cardiovasc. Drugs 10 (2010) 401-412.
[8] A.A. Faruqui, Evaluation of safety and efficacy of telmisartanamlodipine combination in treating hypertension, J. Indian Med.Assoc. 106 (2008) 612-624.
[9] P. Li, Y. Wang,Y. Wang,et al., Determination of telmisartan in human plasma by liquid chromatography-tandem mass spectrometry, J. Chromatogr. B 828 (2005) 126-129.
[10] J.D. Terish, S.S. Kumar, N. Ramesh, et al., Estimation of telmisartan in human plasma by reversed phase liquid chromatography coupled with tandem mass spectrometry—a bioequivalence study application, Der Pharm. Lett. 3 (2011) 289-298.
[11] R.R.Seelam,I.S.Chandiran,K.R.Divi,et al.,Development and validation of high-performance liquid chromatography-tandem mass spectrometric method for simultaneous quantification of telmisartan in human plasma, Int. J. Pharm. Sci. Drug Res.2 (2010) 188-192.
[12] C. Hempena, L. Glasle-Schwarzb, U. Kunzb, et al., Determination of telmisartan in human blood plasma, Part II: liquid chromatography-tandem mass spectrometry method development, comparison to immunoassay and pharmacokinetic study,Anal. Chim. Acta 560 (2006) 41-49.
[13] B. Chen, Y. Liang, Y. Wang, et al., Development and validation of liquid chromatography-mass spectrometry method for the determination of telmisartan in human plasma,Anal.Chim.Acta 540 (2005) 367-373.
[14] V.K. Gupta, R. Jain, O. Lukramc, et al., Simultaneous determination of ramipril, ramiprilat and telmisartan in human plasma using liquid chromatography-tandem mass spectrometry,Talanta 83 (2011) 709-716.
[15] T. Yan, H. Li, L. Deng, et al., Liquid chromatographic-tandem mass spectrometric method for the simultaneous quantitation of telmisartan and hydrochlorothiazide in human plasma,J. Pharm. Biomed. Anal. 48 (2008) 1225-1229.
[16] N. Ferreiros, S. Dresen, R.M. Alonso, et al., Validated quantitation of angiotensin II receptor antagonists (ARA-II) in human plasma by liquid-chromatography-tandem mass spectrometry using minimum sample clean-up and investigation of ion suppression, Ther. Drug Monit. 29 (2007) 824-834.
[17] J.Bhatt,S.Singh,G Subbaiah,et al.,A rapid and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS)method for the estimation of amlodipine in human plasma,Biomed, Chromatogr. 21 (2007) 169-175.
[18] Y. Ma, F. Qin, X. Sun, et al., Determination and pharmacokinetic study of amlodipine in human plasma by ultra performance liquid chromatography-electrospray ionization mass spectrometry, J. Pharm. Biomed. Anal. 43 (2007) 1540-1545.
[19] A. Sirikatitham, K. Panrat, N. Tanmanee, Determination of amlodipine in human plasma by electrospray ionization LC-MS/MS method: validation and its stability studies, Songklanakarin,J. Sci. Technol. 30 (2008) 455-462.
[20] B. Suchanova, R. Kostiainenc, R.A. Ketola, Characterization of the in vitro metabolic profile of amlodipine in rat using liquid chromatography-mass spectrometry, Eur. J. Pharm. Sci. 33(2008) 91-99.
[21] A.K. Sarkar, D. Ghosh, A. Das, et al., Simultaneous determination of metoprolol succinate and amlodipine besylate in human plasma by liquid chromatography-tandem mass spectrometry method and its application in bioequivalence study, J. Chromatogr. B 873 (2008) 77-85.
[22] P. Massaroti, L.A.B. Moraes, M.A.M. Marchioretto, et al.,Development and validation of a selective and robust LC-MS/MS method for quantifying amlodipine in human plasma, Anal.Bioanal. Chem. 382 (2005) 1049-1054.
[23] A.B. Baranda, C.A. Mueller, R.M. Alonso, et al., Quantitative determination of the calcium channel antagonists amlodipine,lercanidipine, nitrendipine, felodipine, and lacidipine in human plasma using liquid chromatography-tandem mass spectrometry,Ther. Drug Monit. 25 (2005) 44-52.
[24] R.V.S. Nirogi, V.N. Kandikere, K. Mudigonda, et al., Sensitive and rapid liquid chromatography/tandem mass spectrometry assay for the quantification of amlodipine in human plasma,Biomed. Chromatogr. 20 (2006) 833-842.
[25] A.V. Ramani, P. Sengupta, R. Mullangi, Development and validation of a highly sensitive and robust LC-ESI-MS/MS method for simultaneous quantitation of simvastatin acid, amlodipine and valsartan in human plasma: application to a clinical pharmacokinetic study, Biomed. Chromatogr. 23 (2009) 615-622.
[26] N.R. Pilli, J.K. Inamadugu, R. Mullangi, et al., Simultaneous determination of atorvastatin, amlodipine, ramipril and benazepril in human plasma by LC-MS/MS and its application to a human pharmacokinetic study, Biomed. Chromatogr. 25 (2011)439-449.
[27] Q. Zou, Y. Zhan, Z. Ge, et al., Liquid chromatography-mass spectrometry method for the determination of amlodipine in human plasma and its application in a bioequivalence study,Arzneimittel-forschung/Drug Res. 59 (2009) 383-391.
[28] US DHHS, FDA, CDER, Guidance for Industry: Bioanalytical Method [8] Validation, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CV), 2001. Available from: 〈http://www/fda.gov/cder/guidance/index.htm〉.
[29] G.F. van Rooyen, D. Badenhorst, K.J. Swart, et al., Determination of carbamazepine and carbamazepine 10,11-epoxide in human plasma by tandem liquid chromatography-mass spectrometry with electrospray ionization, J. Chromatogr. B 769 (2002) 1-7.
 Journal of Pharmaceutical Analysis2012年5期
Journal of Pharmaceutical Analysis2012年5期
- Journal of Pharmaceutical Analysis的其它文章
- Impurity profiling and in-process testing of drugs for injection by fast liquid chromatography
- Authentication and distinction of Shenmai injection with HPLC fingerprint analysis assisted by pattern recognition techniques
- Determination of metronidazole in a rat stomach by HPLC for obtaining basic data of the eradication therapy of Helicobacter pylori
- Bio-analytical method development and validation of Rasagiline by high performance liquid chromatography tandem mass spectrometry detection and its application to pharmacokinetic study
- Monitoring the hydrolyzation of aspirin during the dissolution testing for aspirin delayed-release tablets with a fiber-optic dissolution system
- Kinetic spectrophotometric method for determination of amlodipine besylate in its pharmaceutical tablets
