CaTiO3∶Zn溶胶-凝胶法制备、结构及介电性能
黄万群 张启龙 杨 辉 申乾宏
CaTiO3∶Zn溶胶-凝胶法制备、结构及介电性能
黄万群 张启龙*杨 辉 申乾宏
(浙江大学材料科学与工程学系,杭州 310027)
采用溶胶-凝胶法制备了CaTiO3∶Zn纳米粒子,透射电镜图显示平均粒径为25 nm。Zn的掺杂位置对于陶瓷相组成和烧结特性有很大影响。Ca1-xZnxTiO3和CaTiO3+zZnO样品的Zn以Zn2TiO4相形式存在;而CaZnyTi1-yO3-δ(y=0.01)样品中的Zn进入Ti位形成固溶体,无明显的降温效果,当Zn量增至0.05和0.1时,出现ZnO相。ZnO和Zn2TiO4第二相的存在均能明显促进陶瓷烧结。CaTiO3∶Zn超细粉体可在较低温度下致密烧结(≤1 250℃)。1 250℃烧结的CaZnyTi1-yO3-δ(y=0.01)陶瓷具有较好的介电性能:介电常数 ε=157,品质因数 Q×f=6 819 GHz,谐振频率温度系数 τf=7.51×10-4℃-1。
CaTiO3:Zn;溶胶-凝胶法;纳米粉体;介电性能
0 Introduction
The development of microwave dielectric ceramics resonators for communication systems such as cellular telephones and global positioning systems has been rapidly growing in the past decades[1].In order to reduce circuit/device size,many microwave ceramics with high dielectric constant (εr)such as Ca0.6La0.267TiO3,BaONd2O3-TiO2,CaO-Nd2O3-TiO2and BaO-Gd2O3-TiO2[2-8],have been studied.CaTiO3material is usually used as compensators of the temperature coefficient of resonant frequency(τf)due to its high εr(~170)and large τf(~8×10-4℃-1)[9-13].However,the sintering temperature of pure CaTiO3is very high (≥1 400 ℃)and it has relativelylow Q×f value (~3500 GHz)by the traditional solidstate preparation process.
Sol-gel method has been used for the fabrication of microwave ceramic materials with improved compositional homogeneity, lower densification temperatures,and better dielectric properties[14-17].To the best of our knowledge,there has been no report on the preparation and dielectric properties of CaTiO3∶Zn nanoparticles.
In this work,Zn was introduced into CaTiO3(CT)ceramic as a substitution of A site,B site and as sintering additive with the stoichiometry of Ca1-xZnxTiO3(ZCT),CaZnyTi1-yO3-y(CZT)and CaTiO3+zZnO(CTZ).Well dispersed powders were synthesized by sol-gel method.The effect of the Zn substitution site on sintering temperature and dielectric property was investigated.The sintering temperature was decreased and the Q×f value was improved due to the effect of nano-sized particles and the addition of Zn.
1 Experimental
Powders were sy nthesized by a sol-gel method,using calcium nitrate[Ca(NO3)2·4H2O,99.9%],zinc nitrate[Zn(NO3)2·6H2O,99.9%]and tetrabutyl titanium[Ti(C4H9O)4,99%]as raw materials according to the d esiredstoichiometry:CaTiO3,Ca1-xZnxTiO3,CaZnyTi1-yO3-δ,CaTiO3+zZnO (x=y=z=0.01,0.05,0.1, δ presents oxygen vacancy concentration).Ethanol was used as solvent,PEG1000 was added so as to account for 2wt%of the total solution and nitric acid was used to adjust the pH value to about 4.After 1 h stirring,the light yellow solution became viscous sol.The sol was aged for 10 h until it turned completely a gel.The gel was dried and then treated at 500~800 ℃ for 2 h to get the powder.Then the powder was milled by planetmilling and dried.After that,5wt% of Polyvinyl Alcohol(PVA)was added,and the samples were then pressed into disks under about 80 MPa pressure.The disks were sintered at a temperature ranging from 1 100 to 1 300℃.
Thermal analysis for the dried gel was carried out using a TG-DTA analyzer (German Netzsch Co.STA449C)from room temperature to 1 000℃in air at a heating rate of 10 ℃·min-1.Crystal phases of the nanoparticlesand ceramicswere investigated by Shimadzu XRD-6000 X-raydiffractometerwith a graphite diffracted-beam monochromator for Cu-Kα radiation(λ=0.154 18 nm)at 40 kV and 40 mA,using 0.15 mm receiving slit and scintillation counter as the detector.The 2θ region was in the range of 10°~70°with a step of 0.02°and a scanning speed of 4°·min-1.Transmission electron microscopy (TEM)images were performed on JEM-1230 TEM with an acceleration voltage of 200 kV.Scanning electron microscopy(SEM)was performed on HitachiS4800 SEM atan accelerating voltage of 5 kV.Dielectric constant(εr)and quality factor value(Q×f)at microwave frequency were measured using Hakki-Coleman method and cavity method by vector network analysis (Agilent 8719ET,USA),respectively.
2 Results and discussion
Fig.1 shows the TG and DTA curves of the CaZnyTi1-yO3-δ(y=0.01)xerogel.The appearance of endothermic peaks at 130℃,associated with weight loss between 30 and 150℃in the TG curve is possibly due to the evaporation of residual water and organics absorbed on the surface of the xerogel.A sharp exothermic peak at 160℃accompanied by a sharp weightlossisobserved in theTG curve.This corresponds to the combustion of the xerogel.In this process,it is concluded that decomposition of butoxy,PEG400 and nitrate acid produces a lot of oxygen.The oxygen could accelerate the combustion process and generate a great amount of heat.The second exothermic peak at about 370℃is attributed to the combustion of remaining organics.The endothermic peak at 620 ℃associated with a weight loss of 20%might be due to the decomposition of NO3-.A small endothermic peak appeared at 730℃accompanied with a weight loss of 5%is assigned to the decomposition of CaCO3,which is demonstrated by the analysis of XRD in Fig.2.


XRD patterns of the CaZnyTi1-yO3-δ(y=0.01)xerogel calcined at 500~800 ℃ for 2 h are illustrated in Fig.2.Fig.2(a)shows the presence of orthorhombic perovskite CaTiO3phase as the major phase,along with small peaks corresponding to CaCO3and Ca2Ti2O6.With increasing the temperature from 500℃ to 700℃,the diffraction intensities of CaTiO3and Ca2Ti2O6increase,but the diffraction intensities of CaCO3gradually decrease.At 800 ℃ ,CaCO3and Ca2Ti2O6nearly disappear while intense peaks of CaO appear along with small peaks of Ca2Zn4Ti15O36.Complex reactions happen in this stage including the decomposing of CaCO3,Ca2Ti2O6,and the forming of Ca2Zn4Ti15O36:[18-19]
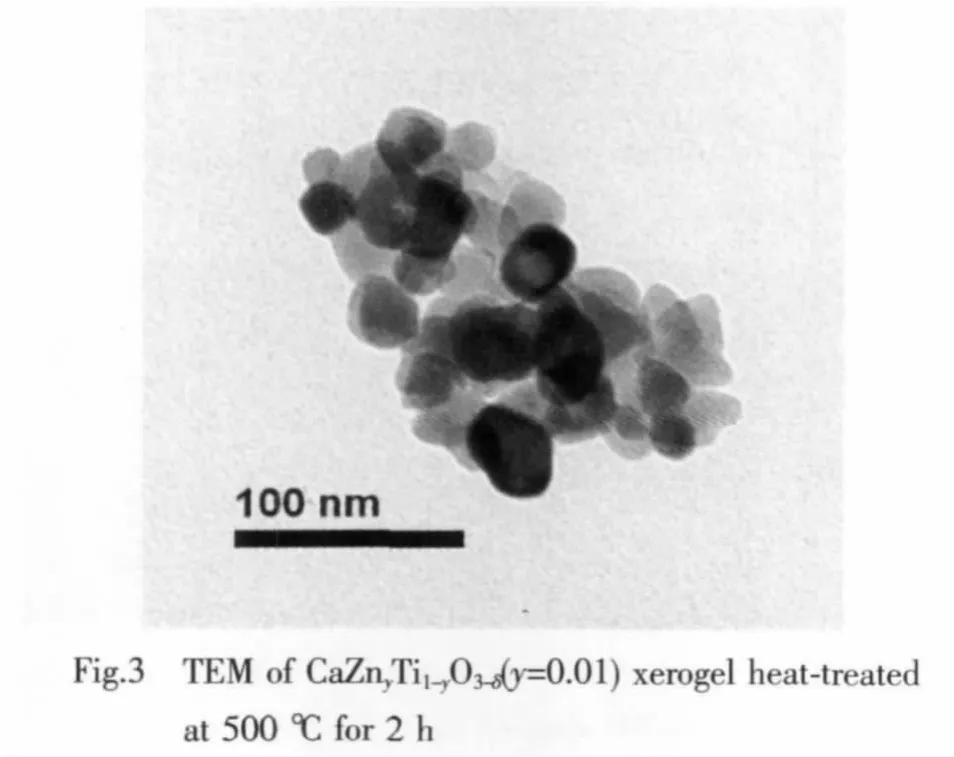

TEM shows that all the samples have similar particle size and shapes.Fig.3 shows the transmission electron micrograph (TEM)of the CaZnyTi1-yO3-δ(y=0.01)xerogel calcined at 500 ℃ for 2 h.It can be seen that the most probable particle size is about 20~30 nm.
Fig.4 illustratesXRD patternsforceramics prepared by nanopowders.Single phase CaTiO3with orthorhombic space group Pmna is obtained in the undoped sample.Except for the orthorhombic perovskite CaTiO3phase as the major phase,the Zn2TiO4phase is formed in the ZCT and CTZ samples,however,a small diffraction peaks from ZnO can be identified in the CZT sample (y≥0.05).There is no evidence of any secondary phases in CaZnyTi1-yO3-δ(y=0.01).

Table 1 shows the unit cell parameters of sintered ceramics.There are no obvious changes on the unit cells of Ca1-xZnxTiO3and CaTiO3+zZnO (x=z=0.05),compared with the pure CaTiO3.However,for CaZnyTi1-yO3-δ,the lattice parameters,especially c and the unit cell volume,show a small increase and there issome indication that the maximum values are obtained at y=0.01.It is clear that a solid solution forms,therefore;possibly the solid solution limit is at y=0.01,which agrees with the reference[20].From the size consideration,it is assumed that Zn substitutes for Ti associated with oxygen vacancies for charge compensation.It also indicates that,for ZCT and CTZ,the ZnO does not obviously substitute for A site or other site,the reaction between the ZnO and TiO2forms the Zn2TiO4phase,which is demonstrated by the analysis of XRD in Fig.4(b)and(d).

Table 1 Unit cell parameters calculated from the XRD
Fig.5 showsthe XRD patternsofceramics CaZnyTi1-yO3-δ(y=0.01)sintered at the different temperatures.Thereisno obviousphase change with increasing the sintering temperature.SEM micrographs of dense CaTiO3∶Zn ceramics are shown in Fig.6(a)~(d).CaZnyTi1-yO3-δ(y=0.01)specimens sintered at 1 150 ℃are not completely dense and a small amount of porosity can be observed (SEM is not shown here).At 1 250 ℃,well-sintered dense ceramics are obtained;the grain size is increased with increasing the sintering temperature.However too high sintering temperature causes the abnormal grain growth,samples sintered at 1 300℃has abnormal grain growth,and parts of the abnormal grain size are larger than 4 μm (SEM is not shown here).Dense ceramics are also obtained for ZnxCa1-xTiO3(x=0.01)and CaTiO3+zZnO (z=0.01)ceramics.However,pure CaTiO3ceramics sintered at 1 250℃is not as dense;pores are obvious as seen from Fig.6(a).It indicates that the dense temperature of ZCT,CZT,and CTZ,is about 1 150℃,1250℃and 1150℃,respectively.The sintering temperature of pure CaTiO3ceramics synthesized by the solid-state reaction method is higher than 1 400℃.In contrast to that,the CaTiO3and CaTiO3∶Zn nanopowders synthesized by the sol-gel method could be densified at lower than 1 250℃.The result indicates that the nanoparticles with high ratio of specific surface area and surface energy contribute to promotethesinteringpropertiesand reduce the sintering temperature.Moreover,the CaTiO3∶Zn has a lower dense temperature than pure CaTiO3.For the ZCT and CTZ ceramics,the added Zn acts as sintering aid,melting at a relatively lower temperature and forms the Zn2TiO4.Zn2TiO4could be sintered at temperatures lower than 1 000℃[21-23],which further improves the sintering characterization.


Table 2 illustrates the dielectric properties of ceramics.For all the samples,the εrachieves the maximum value at the highest relative densities.The εrof CaTiO3doped with Zn is lower than that of pure CaTiO3,and decreases with increasing the Zn content,which is due to the appearance of Zn2TiO4or ZnO with lower εrvalue (≤30)[24-26].Although the Zn2TiO4exhibits a relatively high Q×f value,the ZCT ceramics with dense structure (at 1 150℃)still achieve the lowest Q×f value.The reason for this is not clear at present and will be further studied.For CaZnyTi1-yO3-δ(y=0.01)samples,the εrfirstly increases with the sintering temperatures,reaches the maximum value(~158)at1 200 ℃ ,and then decreaseswith increasing the sintering temperature.The maximum εrvalue of CaZnyTi1-yO3-δ(y=0.01)is lower than CaTiO3duetotheTi siteby Zn substitution and the appearance of oxygen vacancy.It is interesting that the Q×f value increases with the increase of the sintering temperature.The Q×f value can be affected by porosity and grain size[27].Generally,a larger grain size and a smaller grain boundary indicate reduction in dielectric loss.Sample sintered at 1 150 ℃ with low dielectric constant and Q×f value are attributed to a low bulk density.As the sintering temperature increase from 1 150℃ to 1 300℃,the Q×f value increasesdue to grain size augmentand grain boundary reduction.For all samples doped with Zn,the τfvalues are decreased compared with CaTiO3.CaZnyTi1-yO3-δ(y=0.01)ceramics sintered at 1 250 ℃possesses a dielectric constant of 157,a Q×f value of 6 819 GHz and a τfvalue of+7.5×10-4℃-1.

Table 2 Dielectric properties of ceramics
3 Conclusions
The sol-gel method was adopted to synthesize the nano-sized CaTiO3∶Zn particles.The average particle size calculated from TEM micrograph is 25 nm.Zn substitution site has a great influence on phase composition and sintering temperature.The added Zn exists as Zn2TiO4phase for Ca1-xZnxTiO3and CaTiO3+zZnO.However,for CaZnyTi1-yO3-δ(y=0.01),Zn substitutes for Ti site and forms a solid solution,which has no obvious effect on sintering characteristics.As the y value is increased to 0.05 and 0.1,ZnO phase is formed.The existence of ZnO or Zn2TiO4phase can decrease sintering temperature.The ultrafine CaTiO3∶Zn powders can be sintered at relatively lower temperatures (≤1250 ℃)and most of them possess high Q ×f values.CaZnyTi1-yO3-δ(y=0.01)ceramics sintered at 1250℃exhibits good dielectric properties with a dielectric constant of 157 and a Q×f value of 6819 GHz.
[1]Wersing W.Curr.Opin.Solid State Mater.Sci.,1996,1(5):715-731
[2]Li J M,Qiu T,Fan C G,et al.J.Sol-Gel Sci.Technol.,2011,59(3):525-531
[3]Lee W H,Lee Y C.Ceram.Int.2010,36(1):181-185
[4]Fu M S,Liu X Q,Chen X M.J.Eur.Ceram.Soc.,2008,28(3):585-590
[5]Zhang Q L,Sun H P,Yang H.J.Alloys Compd.,2011,509(42):9986-9991
[6]Liang B L,Zheng X H,Tang D P.J.Alloys Compd.,2009,488(1):409-413
[7]Jacob K S,Satheesh R.Mater.Res.Bull.,2009,44(10):2022-2026
[8]Bindra N S,Dalveer K,Singh T K.J.Ceram.Process Res.,2009,10(5):595-599
[9]Wang S F,Wang Y R,Hsu J C.J.Phys.Chem.Solids,2011,72(9):1011-1014
[10]Huang C L,Tseng Y W.J.Am.Ceram.Soc.,2011,94(6):1824-1828
[11]Zhu H,Lu W Z,Lei W.Ceram.Int.,2011,37(5):1515-1519
[12]Huang C L,Chen J Y,Huang G S.J.Alloys Compd.,2010,499(1):48-52
[13]Hsu C H,Ho H A.Mater.Lett.,2010,64(3):396-398
[14]Zhang Q L,Wu F,Yang H.Scripta Mater.,2011,65(9):842-845
[15]Pang L X,Wang H,Zhou D,et al.Mater.Chem.Phys.,2010,123(2/3):727-730
[16]Yue Z,Zhang Y,Gui Z,et al.Appl.Phys.A,2005,80(8):1757-1761
[17]Zhang Q L,Wu F,Yang H,et al.J.Alloys Compd.,2010,508:610-615
[18]ZHANG Qi-Long(张启龙),WANG Huan-Ping(王焕平),YANG Hui(杨辉).Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2009,22(9):1657-1662
[19]Pang L X,Wang H,Chen Y H,et al.J.Mater.Chem.,2009,20(6):528-533
[20]Zhang Q L,Maso N,Liu Y,et al.J.Mater.Chem.,2011,21(34):12894-12900
[21]Ahn C W,Song H C,Nahm S.J.Am.Ceram.Soc.,2006,89(3):921-925
[22]Zhang Q L,Yang H,Zou J L,et al.Mater.Lett.,2005,59(8/9):880-884
[23]Kim H T,Kim S H,Nahm S,et al.J.Am.Ceram.Soc.,1999,82(11):3043-3048
[24]Kim H T,Byun J D,Kim Y.Mater.Res.Bull.,1998,33(6):963-973
[25]Li B,Tang B,Zhang S R,et al.J.Mater.Sci.,2010,45(23):6461-6466
[26]Kim H T,Kim Y.J.Am.Ceram.Soc.,2001,84(5):1081-1086
[27]Perm S J,Alford N M,Templeton A,et al.J.Am.Ceram.Soc.,1997,80(7):1885-1888
Preparation,Structure and Dielectric Properties of CaTiO3∶Zn Ceramics Based on Sol-Gel Technology
HUANG Wan-QunZHANG Qi-Long*YANG HuiSHEN Qian-Hong
(Department of Materials and Engineering,Zhejiang University,Hangzhou,310027,China)
Nano-sized CaTiO3∶Zn particles were synthesized by a sol-gel technique.The average particle size of the powder calculated from TEM micrograph is 25 nm.Zn substitution site has great influence on the phase composition and the sintering temperature of the ceramics.The added Zn exists as Zn2TiO4phase for Ca1-xZnxTiO3and CaTiO3+zZnO.However,for Ca(Zny,Ti1-y)O3-δ(y=0.01),Zn substitutes for Ti site and forms a solid solution,which has no obvious effect on sintering characteristics.When the y value is 0.05 and 0.1,ZnO phase is formed.The existence of ZnO or Zn2TiO4phase can improve the sintering properties.The ultrafine CaTiO3∶Zn powders can be sintered at relatively lower temperatures (≤1250 ℃).CaZnyTi1-yO3-δ(y=0.01)sintered at 1 250 ℃ possesses a dielectric constant εrof 157,a Q×f value of 6819 GHz and a τfvalue of 7.51×10-4℃-1.
CaTiO3:Zn;sol-gel processes;nanoparticles;dielectric properties
TQ174
A
1001-4861(2012)11-2379-06
2011-12-16。收修改稿日期:2012-03-14。
国家科技支撑计划(No.2009BAG12A07);浙江省创新团队(No.2011R09010)资助项目。*
。 E-mail:mse237@zju.edu.cn

