Development of the SCNT Embryo from Butyrolactone I Prematured Bovine Oocytes
Zhou Jia-bo,Zhang Li,Cao Xiao-zhang,Yue Kui-zhong,Liu Di,and Yue Shun-li*
1 College of Life Sciences,Northeast Agricultural University,Harbin 150030,China
2 Livestock Center,Heilongjiang Academy of Agricultural Sciences,Harbin 150086,China
Introduction
Cloning or nuclear transfer using adult somatic cells to derive cloned embryos is a promising new technology with potential applications in both agriculture and regenerative medicine.Somatic cell nuclear transfer (SCNT) has been successfully used in several species(Wani et al.,2010),allowing its commercial application (Meirelles et al.,2010).In most mammalian species studied thus far,the survival rate to birth for cloned blastocysts is only about 1%-5%,compared with 30%-60% birth rate for IVF blastocysts (Yang et al.,2007).A method that enables to increase oocyte quality and the number of oocytes manipulated is needed to improve the efficiency of the SCNT.
Oocyte developmental competence is acquired before maturation while the gamete undergoes several changes at both structural and molecular levels.These have been termed oocyte capacitation and are essential to support embryonic development,whereas the embryo relies on maternal factors (Blondin et al.,1997).Prematured oocytes can be kept arrested at the germinal vesicle (GV) stage of the meiotic division for up to 48 h before being subjected to in vitro maturation (IVM) (Adona et al.,2004).Thus,in vitro prematuration has been suggested as a mean to provide the oocyte with additional time to acquire developmental competence prior to IVM (Kitagawa et al.,1994).As removed from the follicle,oocyte spontaneously resumes the meiotic division progressing into a mature oocyte.Those spontaneous meiosis resumption can be avoided if the oocyte is prematured in the presence of specific cell cycle inhibitors (i.e.,butyrolactone I) after removal from the follicle (Adona et al.,2004).Butyrolactone I is a selective inhibitor of the cyclindependent kinase (cdk) family.It inhibits both cdk2 and cdc2 kinase (Kitagawa et al.,1994).Butyrolactone I has been successfully employed to arrest meiotic progression in several species without harming preimplantation development (De Bem et al.,2011).Prematuration in the presence of low concentration of butyrolactone I arrests over 97% of bovine oocytes at the GV stage of meiosis and has no effects on metaphaseⅡ (MⅡ) formation rate after maturation when compared to controls (Adona et al.,2008).Prematuration has the advantage of enabling more flexibility to the procedure of in vitro embryo production by allowing adjustments to the time of IVM according to the needs of the laboratory (De Bem et al.,2011).This is particularly relevant to the SCNT because it requires a long period of oocyte manipulation.
In this study,we aimed at testing the hypothesis that butyrolactone I prematured oocytes can support embryonic development after the SCNT.
Materials and Methods
Unless stated otherwise all chemicals were purchased from Sigma (Beijing,China).
Preparation of immature oocytes
Bovine ovaries were collected from a local slaughterhouse and transported to the laboratory in phosphatebuffered saline (PBS) at 30℃ within a period of 4 h.Immature oocytes were aspirated from antral follicles (2-6 mm in diameter) with a 19-gauge needle.Cumulus-oocyte complexes with more than three compact layers of cumulus cells were selected,pooled,and rinsed in the HEPES-buffered TCM199 .
Prematuration of oocytes
Maturation medium was 25 mmol • L-1bicarbonatebuffered TCM 199 supplemented with 10% fetal calf serum (FCS) (Gibco),10 mg • mL-1FSH and 1 mg • mL-1estradiol-17β.Butyrolactone I was diluted at 50 mmol • L-1in dimethyl sulfoxide (DMSO) and stored in aliquots at –20℃.Maturation medium was in 100 μL droplets (15-20 oocytes per droplet) under mineral oil for 24 h.In previous experiments,we had already tested working concentration of butyrolactone I in the maturation medium using different concentrations (0,5,10,20,30,40,50,and 100 μmol • L-1,respectively).We found that the majority of the oocytes remained blocked in the GV stage (≥95%) at similar rates irrespective of concentrations (p>0.05),when the meiotic block concentration was only at 10,20,30,40,50,and 100 μmol • L-1,respectively,the oocytes were conducted with butyrolactone I in maturation medium for 24 h.So in this experiment,the oocytes were incubated with butyrolactone I at 10 μmol • L-1.Oocytes were then subjected to IVM as below.
In vitro maturation of oocytes
The oocytes were then cultured for 22 h at 38.5℃ in a humidified atmosphere of 5% CO2in maturation medium.24 h after the beginning of maturation,the oocytes were incubated in HEPES-buffered TCM199 supplemented with 0.5% (w/v) hyaluronidase for 5 min;gentle pipetting was then performed to remove the cumulus cells.Oocytes were then assigned randomly in two groups: parthenogenetic activation and nuclear transfer.
Developmental assessment after parthenogenetic activation
After IVM,oocytes were denuded of cumulus cells and selected for the presence of the first PB.Selected oocytes were chemically activated (at 26 h post-IVM) by incubation in 5 μmol • L-1ionomycin in HEPESbuffered TCM-199 supplemented with 0.1% BSA,0.2 mmol • L-1sodium pyruvate and 10 mg • L-1gentamycin for 5 min,followed by incubation in HEPES-buffered TCM-199 with 3% BSA for 1 min and in 2 mmol • L-16-dimethylaminopurine (6-DMAP) diluted in modified synthetic oviduct fluid (SOFaa) for 4 h.Oocytes were then extensively washed in SOFaa and in vitro cultured in groups of 15-20 in 100 μL droplets of SOFaa on a monolayer of cumulus cells for 192 h.Part of the activated oocytes were,instead of cultured,used for pro-nuclear analyses by staining for 15 min in 10 mg • L-1Hoechst 33342 diluted in phosphate-buffered saline (PBS) with 0.1% (w/v) polyvinyl alcohol (PVA) in the dark.
After washing twice in PBS with 0.1% (w/v) PVA,oocytes were mounted on slides with covers lips using Mounting Medium and visualized on a fluorescence microscope (Leica,Germany).On the other hand,cultured embryos were assessed for developmental rates at day 2 [cleavage at 48 h postparthenogenetic activation (48 hpa)] and day 8 (blastocysts at 192 hpa).Cleavage and blastocyst rates were reported in relation to the number of presumptive zygotes placed in culture.Moreover,blastocysts at day 8 were evaluated for the total number of cells.
Culture and preparation of fibroblast cells for nuclear transfer
The cells used in this study came from fibroblast cultures initiated from an ear of a 5-month-old calf.Frozen cells at passage 4-10 were thawed and the cells were allowed to grow for 5 days in DMEM supplemented with 10% (v/v) FCS until confluency to synchronize at the G0 stage of the cell cycle by contact inhibition.Nuclear donor cells were then dissociated by 5 min of trypsinization at 37℃,resuspended in 1 mL DMEM containing 10% (v/v) FCS and maintained in this medium until nuclear transfer.
Somatic cell nuclear transfer and in vitro culture
Matured oocytes used as recipient ooplasts for the SCNT were obtained after selection for the presence of the first PB as described above,after 18 h post-IVM.These oocytes were incubated in groups of 20-30 in SOFaa containing 10 g • L-1Hoechst and 7.5 mg • L-1cytochalasin B for 15 min.For microsurgery,the group was transferred to a 400 μL droplet of HEPESsupplemented SOFaa with 7.5 mg • L-1cytochalasin B.Nuclear transfer was performed using an inverted microscope (Leica DMI RB;Leica,Wetzlar,Germany) equipped with micromanipulators and microinjectors (Narishige,Tokyo,Japan).The first PB and MⅡ plates were removed by aspiration with a 15 μm inner diameter glass pipette.The first PB and MⅡ removal were confirmed by exposure of the biopsy to ultraviolet light.
Enucleated oocytes were reconstructed by injection of a single fibroblast into the perivitelline space using the same micromanipulation system described above.The resulting couplet was placed in a fusion chamber (ECM2001) filled with electrofusion solution (0.28 mol • L-1mannitol,0.1 mmol • L-1MgSO4,0.5 mmol • L-1HEPES,and 0.05% (w/v) BSA in water) and subjected to one pulse of alternating current (0.05 kV • cm-1for 5 s) and two pulses of continuous current (1.75 kV • cm-1for 45 μs) to promote fusion between the somatic cell and the recipient ooplast.Successfully fused couplets were activated and in vitro cultured as described above.The numbers of blastocyst total nuclei were determined.Parts of the blastocysts were,instead of cultured,used for analyses as the above.
Statistical analysis
Statistical analyses were performed using the SPSS System (Ver.13.0).Data presented as percentage were analyzed using Chi-square or Fisher's exact tests.The numbers of nuclei were analyzed using Student t-test.All experiments were repeated at least three times.Differences with probabilities (p<0.05) were considered significant.The standard error of the mean (SEM) was presented for continuous values.
Results
Supplementation with butyrolactone I during IVPM (in vitro prematuration) improved maturation and cleavage rates.First,we designed an experiment to determine whether the use of butyrolactone I during IVPM or IVM improved oocyte maturation and embryonic development.
To investigate whether butyrolactone I affected the embryonic development,oocytes produced were parthenogenetically activated and in vitro cultured to evaluate developmental rates.
Although cleavage rates were superior when adding butyrolactone I during IVM (79.6.2% vs.59.4%;10 μmol • L-1butyrolactone I vs.control.),developmental rates to blastocyst were unaffected (p>0.05) by the addition of butyrolactone I during both IVM and IVPM (Table 1).

Table1 Effect of butyrolactone I supplementation during in vitro maturation on preimplantation development of parthenogenetic embryos
Blastocyst quality,evaluated by the blastocyst rate and total nucleus numbers (total nucleus numbers,190±22.1vs.195±16.3) (Table 2),did not show (p>0.05) an effect of butyrolactone I regardless of its use during IVM.In summary,these data suggested the use of butyrolactone I during IVPM,but not during IVM,was not harm to oocytes by their cleavage rates after parthenogenetic activation.
To investigate the effect of prematuration on oocyte quality regarding production of cloned embryos,we evaluated the blastocyst rate and total nucleus numbers in cloned embryos.
Cleavage rates were not affected by prematuration when adding butyrolactone I during IVM (62.4 %vs.57.9%;10 μmol • L-1butyrolactone I vs.control.),developmental rates to blastocyst were unaffected (p>0.05) by the addition of butyrolactone I during IVM in cloned embryos (Table 3).

Table2 Effect of butyrolactone I supplementation during in vitro maturation or in vitro prematuration on parthenogenetic blastocysts
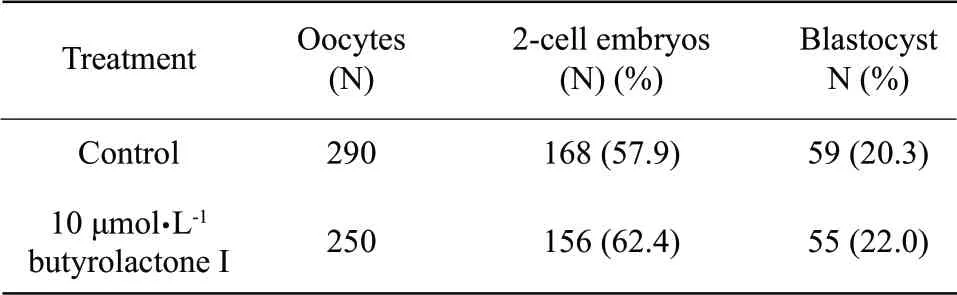
Table3 Effect of butyrolactone I supplementation during in vitro maturation on preimplantation development of the SCNT embryos
Blastocyst quality,evaluated by the blastocyst rate and total nucleus numbers (total nucleus numbers,132±16.5 vs.128±19.4) (Table 4),did not show (p>0.05) an effect of butyrolactone I regardless of its use during IVM.In summary,these data suggested the use of butyrolactone I during IVPM,but not during IVM,was not harm to oocytes by their cleavage rates after the SCNT procedures.

Table4 Effect of prematuration on blastocyst of cloned embryo
These findings indicated that the prematuration of bovine oocytes did not affect oocyte competence and was a viable alternative for the SCNT procedures using standard IVM protocols.
Discussion
In the present study,butyrolactone I was added to prematuration medium at 10 μmol • L-1as our previous results and also suggested by Adona et al (2008).Although we did not observe a positive effect of butyrolactone I during IVM,oocytes prematured in its presence showed higher rates of cleavage.No other significant effects were seen on embryonic development when IVM was performed with butyrolactone I.Although the use of butyrolactone I during prematuration did not lead to better rates of embryo production in our study,cleavage rates were superior to control,indicating an improvement of oocyte developmental competence.
Our aim was to verify the benefits of prematured oocytes as recipient ooplasts for producing cloned blastocyst by the SCNT.Previous studies have shown that prematuration can successfully be used to arrest meiotic division without any negative consequences to oocyte viability (Adona et al.,2008;Coy et al.,2005;Ponderato et al.,2002).In this paper,development to term of pigs produced by IVF has been reported using prematured oocytes (Coy et al.,2005).It seems logically to examine the advantages of prematured oocytes to produce cloned offspring.Our data provided evidence that the use of pre-matured oocytes for producing cloned blastocyst by the SCNT had at least the same efficiency as control oocytes not subjected to prematuration.
This finding indicated that oocytes arrested at the GV stage of meiosis for 24 h could be used as recipient ooplasts for the SCNT without negative consequences to term development,providing an interesting option to the SCNT.Prematuration of oocytes that exceeded the number necessary for a SCNT routine (about 250 immature oocytes) became an interesting option for using surplus oocytes,eliminating the need for another oocyte collection in the following day.
In conclusion,the present study provided a method for laboratories of in vitro embryo production,mainly those working on the SCNT,where prematuration could be used to increase the flexibility of the procedure.
Adona P R,Lima Verde Leal C.2004.Meiotic inhibition with different cyclin-dependent kinase inhibitors in bovine oocytes and its effects on maturation and embryo development.Zygote,12(3): 197-204.
Adona P R,Pires P R,Quetglas M D,et al.2008.Prematuration of bovine oocytes with butyrolactone I: effects on meiosis progression,cytoskeleton,organelle distribution and embryo development.Anim Reprod Sci,108(1/2): 49-65.
Blondin P,Coenen K,Guilbault L A,et al.1997.In vitro production of bovine embryos: developmental competence is acquired before maturation.Theriogenology,47(5): 1061-1075.
Coy P,Romar R,Payton R R,et al.2005.Maintenance of meiotic arrest in bovine oocytes using the S-enantiomer of roscovitine: effects on maturation,fertilization and subsequent embryo development in vitro.Reproduction,129(1): 19-26.
De Bem T H,Chiaratti M R,Rochetti R,et al.2011.Viable calves produced by somatic cell nuclear transfer using meiotic-blocked oocytes.Cell Reprogram,13(5): 419-429.
Kitagawa M,Higashi H,Takahashi I S,et al.1994.A cyclin-dependent kinase inhibitor,butyrolactone I,inhibits phosphorylation of RB protein and cell cycle progression.Oncogene,9(9): 2549-2557.
Meirelles F V,Birgel E H,Perecin F,et al.2010.Delivery of cloned offspring: experience in Zebu cattle (Bos indicus).Reprod Fertil Dev,22(1): 88-97.
Ponderato N,Crotti G,Turini P,et al.2002.Embryonic and foetal development of bovine oocytes treated with a combination of butyrolactone I and roscovitine in an enriched medium prior to IVM and IVF.Mol Reprod Dev,62(4): 513-518.
Wani N A,Wernery U,Hassan F A,et al.2010.Production of the first cloned camel by somatic cell nuclear transfer.Biol Reprod,82(2): 373-379.
Yang X,Smith S L,Tian X C,et al.2007.Nuclear reprogramming of cloned embryos and its implications for therapeutic cloning.Nat Genet,39(3): 295-302.
 Journal of Northeast Agricultural University(English Edition)2012年2期
Journal of Northeast Agricultural University(English Edition)2012年2期
- Journal of Northeast Agricultural University(English Edition)的其它文章
- Effects of Acute and Chronic Cold Stress on Antioxidant Function in Intestinal Tracts of Chickens
