A Study on Stoichiometry of Complexes of Tributyl Phosphate and Methyl Isobutyl Ketone with Lithium in the Presence of FeCl3*
ZHOU Zhiyong (周智勇), QIN Wei (秦炜)**, FEI Weiyang (费维扬) and LI Yigui (李以圭)
State Key Laboratory of Chemical Engineering, Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
1 INTRODUCTION
Lithium is the lightest and the most important metal element. Its metal and compounds are widely used in military industry, lithium alloy, lithium battery,atomic energy, aerospace, metallurgy, ceramics, glass,medicine,etc[1-4]. Since lithium battery has become more and more important as a potential new energy carrier in the world, increasingly importance has been attached to the production of lithium metal and compounds. Most lithium products are produced currently from lithium ores in China. Recent research has focused on extracting lithium from brine sources, by techniques such as adsorption by acid and sodium amberlites resin [5], extraction using supported liquid membranes [6], nanofiltration [7], and ion-sieve adsorption with novel nanocrystal MnO2[8, 9],etc.
Liquid-liquid extraction is an efficient, economical and environment benign method for the separation of carboxylic acids, amino acids, metal ions, amines,phenols,etc. from dilute solutions, and receives increasing attentions [10-16]. It has been intensively studied as an important technology for retrieving lithium from brine sources, which uses the specific affinity to implement the extraction of lithium [17]. The most typical extraction system is TBP (tributyl phosphate)/kerosene-FeCl3, especially for brine sources with high Mg-Li ratio. In this system FeCl3plays a role as coextracting agent, MgCl2which is the typical component in brine sources provides chloride ion environment of high concentration, and both of them make great contribution to extraction of lithium [18-20].
Tributyl phosphate (TBP) is one of the most popular neutral organophosphorus extractants, methyl isobutyl ketone (MIBK) is a typical middle-boiling point solvent. In this study, TBP and MIBK were selected as extractants for lithium extraction. The liquid-liquid extraction reactions for lithium extraction were proposed,and the composition of the complexes for TBP and MIBK with lithium were determined by regressing the experimental data, so as to provide the basis for technological design of practical lithium extraction process.
2 EXPERIMENTAL
2.1 Chemicals
The properties of the reagent, LiCl (Beijing Yili Fine Chemical Co., purity > 97%), MgCl2(Beijing Modern Eastern Finechemical Co., purity > 98%), and FeCl3(Tianjin Yongda Chemical Reagent Co., purity >99%), are listed in Table 1. The physical properties of the extractants, TBP [Beijing Chemical Reagent Plant,purity >99 (by mass)], and MIBK [Beijing Yili Fine Chemical Co., purity >99% (by mass)] are listed in Table 2.
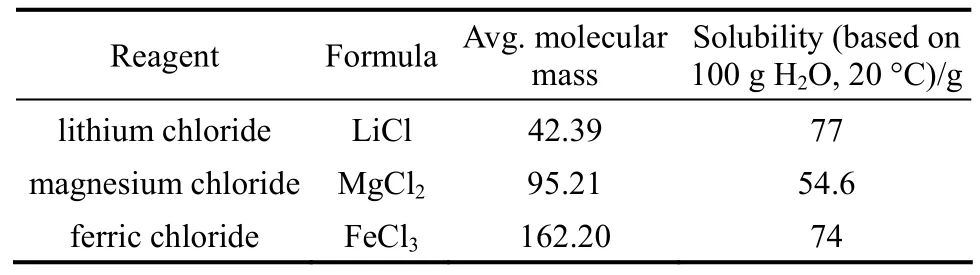
Table 1 Physical properties of salts [21]
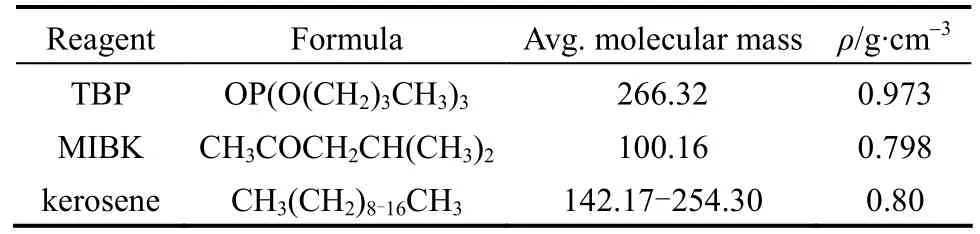
Table 2 Physical properties of extractant and diluents [21]
2.2 Extraction experiments
All extraction experiments were conducted in 50 ml flasks at (25±2) °C. The solvent (10 ml) and 10 ml of the mixed aqueous solution of LiCl (0.025-0.05 mol·L-1), MgCl2(3.5-4 mol·L-1) and FeCl3(0.025-0.09 mol·L-1) were added to the flask. The flasks containing the mixture were shaken manually for 10 min and then left to stand to equilibrium for 30 min, followed by separating the two phases. Preliminary experiments showed that the equilibrium can be reached in 10 min in all experimental solution systems. Aqueous-phase samples were taken from the bottom layer (aqueous phase) using a syringe with a long needle for determining the equilibrium composition.
2.3 Sample analysis
The aqueous samples were analyzed for lithium concentration by using a Z-5000 atomic absorption spectrometer (Hitachi, Japan). The lithium concentration in the organic phase was calculated by the material balance. Preliminary stripping experiment of the organic phase indicated that the deviation of calculated lithium concentration was within ±3%.
3 RESULTS AND DISCUSSION
3.1 Liquid-liquid extraction equilibrium and chemical reaction
As is well known, a high concentration of Cl-(>6 mol·L-1) is required to achieve complete extraction of Fe(III) with TBP and MIBK. HFeCl4can be formed as the complex which has special binding force with TBP and MIBK [22, 23]. In this work, HFeCl4and TBP,MIBK systems are selected to recover lithium from brine sources of high Mg-Li ratio. FeCl3and high concentration of chloride ion play important roles in extracting lithium, which can promote the extraction reaction for lithium.
In order to further understand the characteristics of liquid-liquid extraction equilibrium, the determination of the complexes of TBP and MIBK with lithium is very important which can be used to predict and design extraction process. On the basis of the extraction characteristics and mechanism analyses [20], the extraction reaction in this system is proposed as follows:

whereSstands for extractants, and aqueous phase and organic phase are denoted by the subscript a and o,respectively.Kexis apparent equilibrium constant. The stoichiometric coefficient of Fe(III) in the complex of lithium is denoted byx, and that of TBP or MIBK isz.
Four forms of FeCl3, FeCl2+,2FeCl+, FeCl3andexist in aqueous phase. The dissociation reactions and species conservation are shown as follows:

whereβistands for dissociation constant of FeCl2+,.
The distribution coefficient,D, is defined as the total molar concentration of lithium in organic phase divided by that in aqueous phase:

whereMis only related to the concentration of chloride ion in aqueous phase. Using Eqs. (8) and (9), Eq.(2) can be simplified:
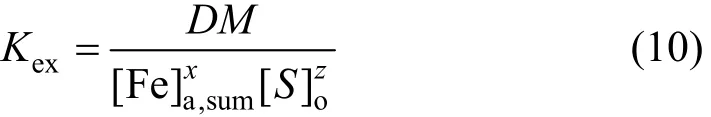
and its log form is
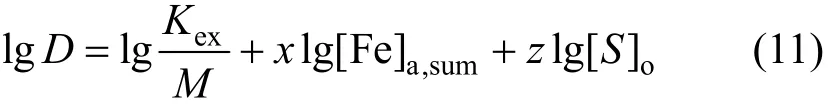
Thus,xandzcan be evaluated from Eq. (11) by regressing experimental data.
3.2 Stoichiometry of lithium and Fe(Ⅲ) with TBP and MIBK in complex
Effect of Fe(III) concentration on-lithium extraction at constant concentrations of Cl and TBP or MIBK are shown in Figs. 1 and 2, respectively. Under the above conditions,and l g [S]oshould be constant, andxin Eq. (11) can be determined as the slope of the fitting line. All fitting lines of lgDLiversuslg[Fe]a,sumrepresent the experimental data well, and their slopes are close to 1. Therefore,xin Eq. (11) is determined to be 1 with either TBP or MIBK as extractant for lithium extraction, and the complex formed is LiFeCl4·zTBP and LiFeCl4·zMIBK, respectively.

Figure 1 Effect of Fe(III) ion concentration on the extraction of lithium with TBP as extractant at constant concentration of Cl- and TBP ([Cl-]=7 mol·L-1, [Li+]=0.05 mol·L-1)1—[TBP]=0.44 mol·L-1; 2—[TBP]=0.52 mol·L-1; 3—[TBP]=0.59 mol·L-1; 4—[TBP]=0.74 mol·L-1

Figure 2 Effect of Fe(III) ion concentration on the extraction of lithium with MIBK as extractant at constant concentration of Cl- and MIBK ([Cl-]=7 mol·L-1)1 — [Li+]=0.025 mol·L-1, [MIBK]=5.56 mol·L-1; 2 —[Li+]=0.05 mol·L-1, [MIBK]=5.56 mol·L-1; 3—[Li+]=0.05 mol·L-1, [MIBK]=7.15 mol·L-1; 4 — [Li+]=0.05 mol·L-1,[MIBK]=7.95 mol·L-1
Effect of TBP concentration on lithium extraction at constant concentration of Cl-and Fe3+are shown in Fig. 3,and l g [Fe]a,sumshould be constant,andzin Eq. (11) can be determined as the slope of the fitting line for plotting the lgDLito lg[S]o. The results showed that the slopes of all fitting lines of lgDLito lg[S]oare close to 1 at constant Cl-and Fe3+concentrations. It indicates thatzin Eq. (11) is 1 with TBP as extractant for lithium extraction. Therefore, the complex formation is LiFeCl4·TBP with TBP as extractant for lithium extraction.
3.3 Stoichiometry of lithium with TBP in complex
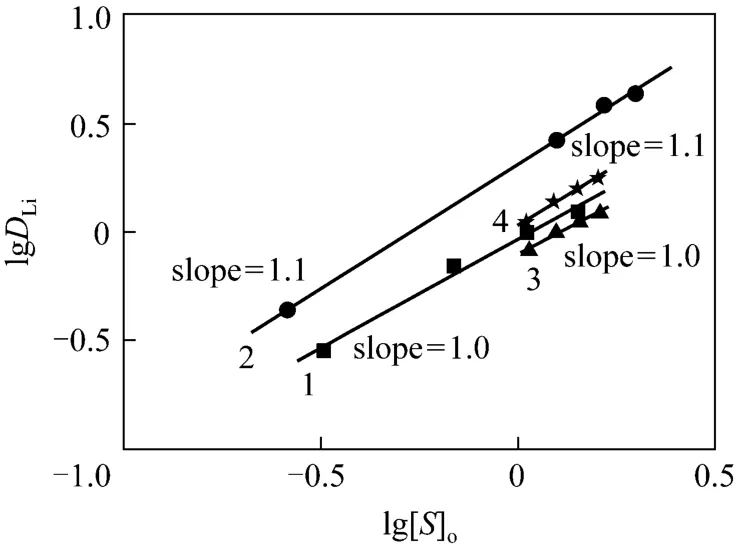
Figure 3 Effect of TBP concentration on the extraction of lithium at constant concentration of Cl- and Fe3+ ([Li+]=0.05 mol·L-1)1—[Cl-]=8 mol·L-1, [Fe3+]=0.05 mol·L-1; 2—[Cl-]=8 mol·L-1, [Fe3+]=0.09 mol·L-1; 3—[Cl-]=7 mol·L-1, [Fe3+]=0.05 mol·L-1; 4—[Cl-]=7 mol·L-1, [Fe3+]=0.075 mol·L-1
Ion-pair extraction may exist between TBP, Li+and4FeCl-, because there is effective functional group P O in TBP, and4FeCl-can be formed at high concentration environment of chloride ion. Large cation[TBP·Li]+can be formed, which has strong cation-anion force with large anion4FeCl-. It may be the reason why FeCl3and high concentration of chloride ion promote lithium extraction.
3.4 Stoichiometry of lithium with MIBK in complex
Similarly, stoichiometry of lithium with MIBK in complex can be obtained by plotting the lgDLito lg[S]oat constant Cl-and Fe3+concentrations. As shown in Fig. 4, it can be found that all lines fit the experimental data well, and the slopes of all fitting lines of lgDLito lg[S]owith MIBK as extractant are close to 2. Therefore,zin Eq. (11) can be determined as 2 with MIBK as extractant for lithium extraction,namely, the formed complex is LiFeCl4·2MIBK.
Also, ion-pair extraction may exist between MIBK, Li+and4FeCl-, as there is an effective functional group C O in MIBK.
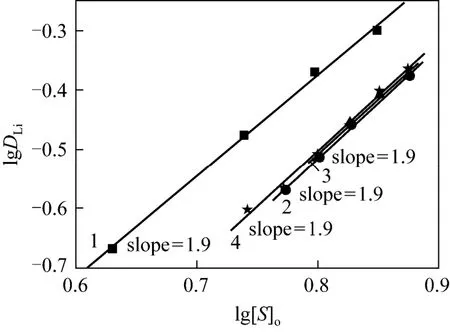
Figure 4 Effect of MIBK concentration on the extraction of lithium at constant concentration of Cl- and Fe3+1—[Li+]=0.05 mol·L-1, [Cl-] = 8 mol·L-1, [Fe3+]=0.05 mol·L-1; 2 — [Li+]=0.025 mol·L-1, [Cl-]=7 mol·L-1,[Fe3+]=0.025 mol·L-1; 3—[Li+]=0.025 mol·L-1, [Cl-]=7 mol·L-1, [Fe3+]=0.0375 mol·L-1; 4—[Li+]=0.025 mol·L-1,[Cl-]=7 mol·L-1, [Fe3+]=0.05 mol·L-1
4 CONCLUSIONS
The extractive reactions for lithium extracted by TBP in kerosene and MIBK in kerosene with FeCl3were proposed. FeCl3plays the role as a coextracting agent which promotes the extractive reaction for lithium. The stoichiometry of the formed complex of lithium and tetrachloroferrate(III) is 1 with either TBP or MIBK as extractant. The complex formed of lithium with TBP and MIBK is LiFeCl4·TBP and LiFeCl4·2MIBK, respectively, at the specific concentration range of all reagents used in this work.
1 Jiang, M., “The application of lithium and lithium compounds”,Inorg. Chem. Ind., 9, 32-36 (1983). (in Chinese)
2 You, Q.Z., “The application of lithium and lithium compounds in medicine field”, World Nonferrous Metal, 12, 41-43 (1997). (in Chinese)
3 Johonson, F.N., “The early history of lithium therapy”, Lithium Cur.Appl. Med. Sci. Technol., 1, 337-338 (1985).
4 Li, H.Y., Zhai, X.J., Fu, Y., “Microwave processing and constructive character of LiCoO2cathode materials for lithium ion batteries”,Journal of Molecular Science, 18 (4), 199-203 (2002). (in Chinese)
5 Navarrete-Guijosa, A., Navarrete-Casas, R., Valenzuela-Calahorro,C., López-González, J.D., García-Rodríguez, A., “Lithium adsorption by acid and sodium amberlite”, J. Colloid Interf. Sci., 264, 60-66(2003).
6 Ma, P., Chen, X.D., Hossain, M.M., “Lithium extraction from a multicomponent mixture using supported liquid membranes”, Sep. Sci.Technol., 35 (15), 2513-2533 (2000).
7 Ma, P.H., Deng, X.C., Wen, X.M., “Nano-filtration method, for separating magnesium and enriching lithium from salt lake brine”,CN Pat., 1542147 (2004). (in Chinese)
8 Zhang, Q.H., Sun, S.Y., Li, S.P., Jiang, H., Yu, J.G., “Adsorption of lithium ions on novel nanocrystalMnO2”, Chem. Eng. Sci., 62,4869-4874 (2007).
9 Feng, Q+., Miyai, Y., Kanoh, H., Ooi, K., “Li+and Mg2+extraction and Li insertion reactions with LiMg0.5Mn1.5O4spinel in the aqueous phase”, Chem. Mater., 5, 311-316 (1993).
10 Kertes, A.S., King, C.J., “Extraction chemistry of fermentation product carboxylic acids”, Biotechnol Bioeng., 28, 269-282 (1986).
11 King, C.J., “Amine-based systems for carboxylic acid recovery: tertiary amines and the proper choice of diluent allow extraction and recovery from water”, Chemtech., 5, 285-291 (1992).
12 Hartl, J., Marr, R., “Extraction processes for bioproduct separation”,Sep. Sci. Technol., 28, 805-819 (1993).
13 Juang, R.S., Huang, R.H., “Comparison of extraction equilibria of succinic and tartaric acids from aqueous solutions with tri-n-octylamine”, Ind. Eng. Chem. Res., 35, 1944-1950 (1996).
14 Chen, Y.H., Yan, J.Y., Li, Y.K., Feng, H.Z., Yao, B.L., Sheng, H.Y.,“Studies on the extraction chemistry of alkali metals—II. The mechanism of the extraction of lithium by the neutral synergistic system of sudan I”, Chinese J. Org. Chem., 4, 257-262 (1982). (in Chinese)
15 Zhou, Z.Y., Qin, W., Dai, Y.Y., “Extraction equilibria of trimellitic and [1,1’-biphenyl]- 2,2’-dicarboxylic acid with 1-octanol, 50%TBP,and 10%TRPO in kerosene”, Chin. J. Chem. Eng., 16 (6), 867-870(2008).
16 Zhou, Z.Y., Qin, W., “Extraction properties of phthalic acid and aromatic polycarboxylic acids using various solvents”, J. Chem.Technol. Biot., 86 (4), 492-496 (2011).
17 Zhang, J.C., Wang, M., Dai, J., “Summarization of the lithium extraction system”, J. Salt Lake Res., 13 (1), 42-48 (2005). (in Chinese)
18 Sun, X.L., Chen, B.Z., Xu, H., Shi, X.C., “Extraction of lithium from bittern”, J. Cent. South Univ. (Sci. Technol.), 38 (2), 262-266(2007). (in Chinese)
19 Zhu, S.L., Piao, X.L., Gou, Z.M., “Extraction of lithium from brine with neutral organophosphorous solvents”, J. Tsinghua Univ. (Sci.Technol.), 40 (10), 47-50 (2000). (in Chinese)
20 Zhou, Z.Y., Qin, W., Fei, W.Y., “Extraction equilibria of lithium with tributyl phosphate in three diluents”, J. Chem. Eng. Data., 56,3518-3522 (2011).
21 Dean, J.A., Lange’s Handbook of Chemistry, McGraw-Hill, New York (1999).
22 Reddy, B.R., Sarma Bhaskara, P.V.R., “Extraction of iron(III) at macro-level concentrations using TBP, MIBK and their mixtures”,Hydrometallurgy, 43, 299-306 (1996).
23 Saji, J., Reddy, M.L.P., “Liquid-liquid extraction separation of iron(III) from titania wastes using TBP-MIBK mixed solvent system”,Hydrometallurgy, 61, 81-87 (2001).
 Chinese Journal of Chemical Engineering2012年1期
Chinese Journal of Chemical Engineering2012年1期
- Chinese Journal of Chemical Engineering的其它文章
- Festschrift in Honor of the 90thBirthday of Prof. Chen Jiayong
- Ternary System of Fe-based Ionic Liquid, Ethanol and Water for Wet Flue Gas Desulfurization*
- The Research Progress of CO2Capture with Ionic Liquids*
- Synthesis of PGMA Microspheres with Amino Groups for High-capacity Adsorption of Cr(VI) by Cerium Initiated Graft Polymerization*
- Solvothermal Synthesis and Optical Performance of One-dimensional Strontium Hydroxyapatite Nanorod*
- Effects of Additives and Coagulant Temperature on Fabrication of High Performance PVDF/Pluronic F127 Blend Hollow Fiber Membranes via Nonsolvent Induced Phase Separation
