Effects of Pre-oxidation Conditions on Adsorption Performance of Activated Carbon Fibers
CAI Yu-lin(蔡玉琳),XU Shan-qing(徐山青),DING Xin(丁 辛),3*
1 Key Laboratory of Textile Science&Technology,Ministry of Education of China,Donghua University,Shanghai 201620,China
2 School of Textile and Clothing,Nantong University,Nantong 226019,China
3 College of Textiles,Donghua University,Shanghai 201620,China
Introduction
Polyacrylonitrile (PAN),one of the predominant precursors for preparing activated carbon fibers(ACFs),can be electrospun into continuous and aligned fibers because of its excellent electrospinnability[1].PAN fibers can be converted into ACFs byoxidative stabilization and carbonization or activation.Compared with traditional methods such as vapor growth and plasma enhanced chemicalvapordeposition,electrospinning is much simpler and more cost effective.
Pre-oxidative stabilization is an essentialand timeconsuming step in converting PAN fibers to high-performance ACFs.During oxidation process,intramolecular cyclization reactions and intermolecular cross-link reactions happen between the linear PAN molecules to form ladder-shaped structure,allowing oxidized fibers withstanding high temperature processing[2]. Previous researches revealed thatthe preoxidation process was greatly influenced by several variables such as the pre-oxidation temperature,its heating rate,preoxidation time,and air flow rate[3-5].To the present,studies on this area mainly focus on the effects of oxidation conditions on the structure and properties of pre-oxidative fibers[6,7],few on the adsorption capability of final ACFs,which is in fact the most important property of the fibers[8].In the present work,PAN fiber webs were prepared by electrospinning.The effects of pre-oxidation conditions were examined,such as temperature,heating rate,and treatment time on the structure and performance of pre-oxidative fibers.The dynamic benzene adsorption capacity ofthe ACFs prepared under different oxidation conditions was measured and evaluated to show the adsorption performance of the fibers.
1 Experimental
1.1 Materials
PAN powder(average molecular weight of 80 000 g/mol)was purchased from Shanghai Synthetic Fiber Institute,China.N,N-dimethyformamide(DMF,945.0-950.0g/L)was purchased from Nanjing Chemical Reagent Co.,Ltd.KH2PO4from Xilong Chemicals Co.,Ltd.,Guangzhou,China.was used as a chemical activation agent.Benzene was purchased from Jiangsu Qiangsheng Co.,Ltd.,China.
1.2 Preparation of ACFs
To obtain PAN fiber webs,PAN solution(12%)for electronspinning was prepared by dissolving PAN powder in the solvent DMF.In spinning a micro flow pump(WZS-50F6,Zhejiang University Medical Instrument Co.,Ltd.)was used and the inner diameter of the needle was 1.2 mm.Other spinning parameters include:voltage 20 kV,feeding rate 2 mL/h,needle-collector distance 20 cm.
PAN fiber webs were then oxidized in a furnace(704-2 double track microperfusion pump,ShanghaiHengchang Instruments).The fiberwebswere heated from room temperature to a temperature(190-270℃)with a chosen heating rate(0.25-1.25 ℃·min-1)in an air atmosphere,and then held at the temperature in different time durations(30-150 min)for oxidation.
For activation,the pre-oxidative fiber webs were immersed into KH2PO4(1.0%)for 10 h and then dried in an oven at 120℃ for 4 h.After that,the fiber webs were activated in a furnace(SX-18-10Q Furnace,Wanshi Electric Furnace Plant,Wuxi,China)by heating up to 500℃ at a rate of 5℃·min-1in a nitrogen atmosphere,held at 500℃ for 30 min,and cooled down to room temperature.Finally,the activated fibers,the ACFs were washed with distilled water for several times and dried in an oven at 120℃ for 4 h.
1.3 Measurement of thermal properties
A scanning electron microscope(SEM,KY KY-2800B,KYKY Technology Development Ltd.,Beijing,China)was used to visualize the morphology of the fiber webs.The diameters of prepared fibers were measured by HJ2000 image analysis software.
The thermal properties were measured by a differential scanning calorimeter(DSC,Netzsch,STA449F3Jupiter,Germany).The samples were heated from 25℃ to 350℃ in a nitrogen atmosphere at a heating rate of 10℃·min-1,held at 350℃ for 10 min,and then cooled down to 25℃ at the same rate as the heating up.
To investigate the changes in the functional groups of fibers through the oxidation and activation,FTIR analysis(Thermo-Nicolet,Avatar 370 FTIR)was used.The FTIR spectra of the fiber samples were measured by using the KBr pellets method.All IR spectra were measured at 8 cm-1resolutions.
1.4 Adsorption efficiency of benzene
Dynamic benzene adsorption of ACFs was measured at room temperature by using a self-made system[9],shown in Fig.1.The dry and clean air by an air compressor((1240 ±100)mL·min-1)was introduced into the benzene generator,in which the air was mixed with benzene gas.The mixed gas passed through the adsorption tube,in which about 0.1 g sample of dry ACFs was located.The weight of the samples was recorded and theadsorption capacity (mg · g-1)was calculated:

where m1and m2are the dry weights of ACFs before and after benzene adsorption.

2 Results and Discussion
2.1 The characteristics of pre-oxidative fibers
To explore the effect of pre-oxidation conditions on the adsorption performance of ACFs,SEM,DSC,and FTIR were used to characterize the pre-oxidative fibers.
The morphology of the fiber webs is shown in Fig.2,and the average diameters and standard deviation of the fibers were listed in Table 1.

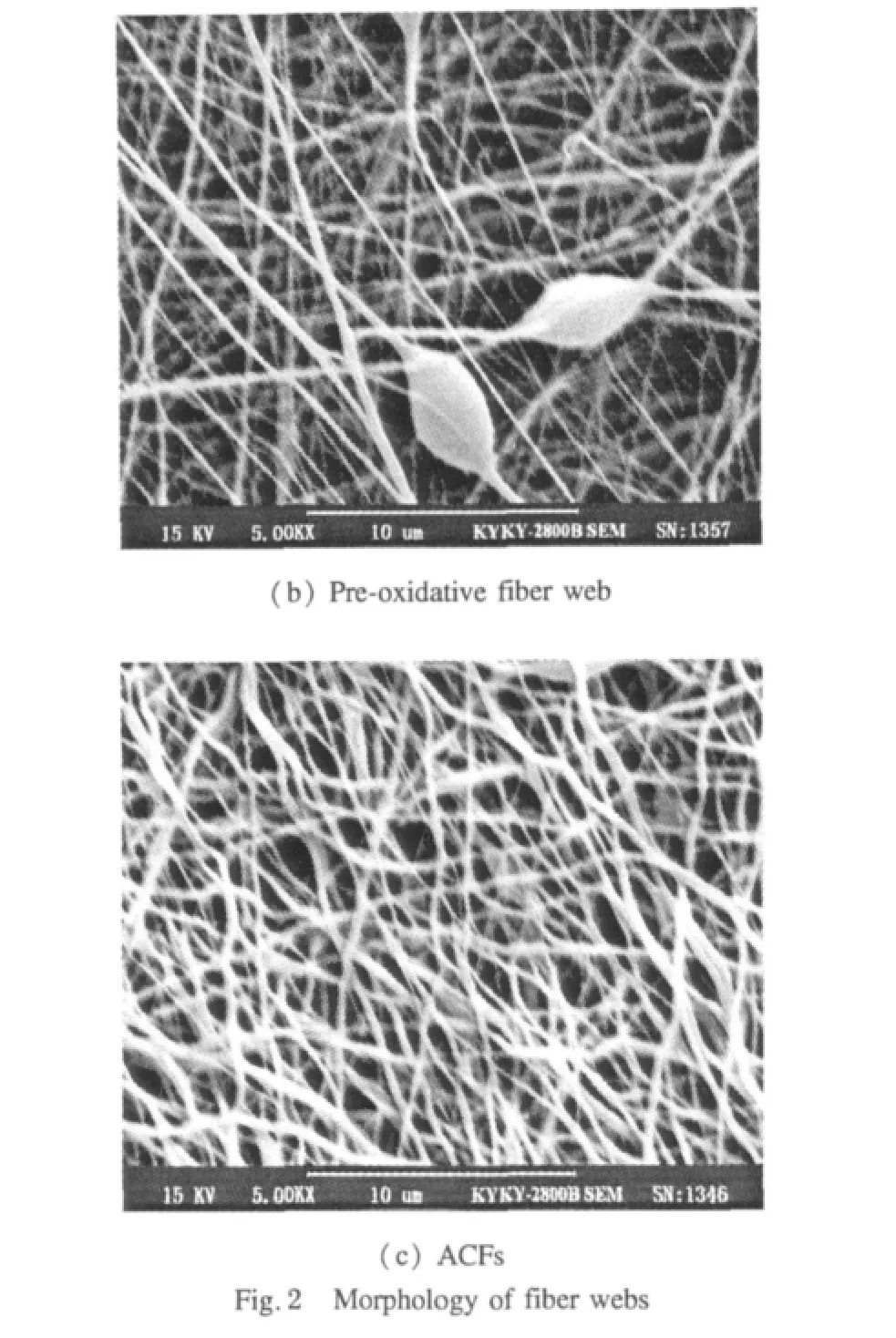
In Fig.2(a)the PAN fiber web shows a fine structure.However,some beads can be noticed in the oxidized fiber web in Fig.2(b),which may result from the high temperature in the pre-oxidation process. Compared with PAN fibers,fiber diameterdecreased after oxidization but increased after activation as seen in Table 1.It could be expected that,after oxidation,more condense ladder-shaped structures were formed and some small molecules were released during the oxidation process,resulting in a reduced fiber diameter.Because of the high temperature during the activation process,the oxidized fibers could melt and then combine together,so that the fiber diameter would increase.In this context,thanks to the very high specific surface of electrospun PAN fiber web,ACFs showed improved adsorption performance when compared with normal activated carbon fibers prepared under the same process.

Table 1 Fiber diameter in fiber webs
DSC curve of PAN fibers in Fig.3 shows a sharp,narrow exothermic peak from 230℃ to 310℃.This exotherm is associated with a nitrile cyclization reaction which leads to a material comprising segments of short ladder molecules linked by unchanged PAN chain sequences[10].The dash DSC curves in Fig.3 are for pre-oxidative fiber(temperature 270 ℃,heating rate 0.5 ℃·min-1,treatment time 30 min),in which no significant exothermic peak is observed.This indicates that the thermoduric ladder-shaped structure has been formed in the pre-oxidation process.

Fig.3 DSC curves of PAN and pre-oxidative fibers
Figure 4 shows the FTIR spectra of PAN fibers,preoxidative fibers obtained at various oxidation temperatures,and ACFs.The peaks at 2 240 cm-1(—C =N)and 1 730 cm-1(—C =O)disappear after oxidization.However,the peaks appear around 1 600 cm-1(—C =N)and 810 cm-1(—C =C—H),which indicate the cyclization and dehydrogenation reactions.It can be confirmed that,during oxidation,the linear macromolecules of PAN fibers are converted into a laddershaped structure to strengthen the thermal property of the fibers[5].A new peak at 1 120 cm-1is attributed to the —C—N group in ACFs,which forms during the activation process.

Fig.4 FTIR spectra of fibers at different processing stages:PAN fibers;pre-oxidative fibers under different temperatures held for 30 min in air;and ACFs(pre-oxidized at 250 ℃,0.5℃ ·min-1,30 min)activated under 500℃in N2
Dalton and Belousova et al.[11,12]investigated the thermal stabilization of PAN fiber by FTIR and defined the extent of reaction:
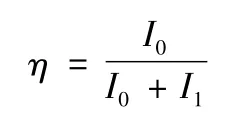
where I1and I0are the height of the peaks at 2 240 cm-1(—C≡N)and 1 600 cm-1(—C =N),respectively.
Figure 5 shows the calculated extent of reaction of preoxidative fibers under different pre-oxidation conditions.The extent of reaction becomes greater with increasing oxidation temperature,heating rate,and treatment time.It indicated that more PAN molecules converted into condensed and thermotolerant ladder-shaped structures.

2.2 Benzene adsorption capacity of ACFs
As shown in Fig.6,ACFs obtained under different pre-oxidative temperatures have a similar adsorption trend,i.e.,most of the adsorption processes take place at the first 10 min due to the fact that the abundant active functional groups are on the fiber surface,where the interaction happens in a short time.It is a kind of micro-pore filling,so that the adsorption speed is fast[13].The equilibrium adsorption capacity of benzene by ACFs is in an order of 230℃>210℃>250℃>270℃>190℃.The results indicate that the pre-oxidation temperature affects the adsorption performance.Because benzene adsorption capacity of ACFs shows a maximum value around the preoxidation temperature of 230℃,it means a moderate extent of reaction is probably favorable for the pre-oxidative fibers to develop active functional groups and micro-pore structures,which can improve the adsorption efficiency of ACFs[14].When the pre-oxidation temperature was heating up to 270℃,the preoxidative fibers formed more thermal-stable structures,which retarded the formation of micro-pore structure and functional groups during the activation process.While the pre-oxidation temperature was 190℃,the thermal stability of pre-oxidative fibers was too poor,leading to the collapse of pore structure under high temperature.

Fig.6 Benzene adsorption capacity of ACFs under different pre-oxidative temperatures
Benzene adsorption capacity ofACFsobtained with different heating rates in the pre-oxidation process is shown in Fig.7.The adsorption capacity increases with increasing heating rate when the rate is smaller than 0.75 ℃ ·min-1and the capacity reaches equilibrium after 20 min.It indicates that the ACFs with smaller heating rates than 0.75 ℃ ·min-1have transitional pores resulting in longer adsorption pathways and slower adsorption rate.Between 0.75 and 1.0 ℃ ·min-1the capacity reaches the maximum,and then the benzene adsorption capacity reduces with heating rate increasing.However,the capacity reaching equilibrium requires only 10 min,and the adsorption rate is much faster than that of fibers obtained from the smaller heating rates in the pre-oxidative process.By referring to the effects of heating rate on extent of reaction in Fig.5,it would be understandable that the more condensed ladder-shaped structures in pre-oxidative fibers hinder the pore widening process during the activation process.
Figure 8 depicts the benzene adsorption capacity of ACFs obtained with different treatment time in the pre-oxidative process.It is noticed that the adsorption capacity is decreasing with increase of treatment time.This is because that the inner structure of pre-oxidative fibers becomes more stabilized and more thermo-tolerant with increasing treatment time.This structure hinders the formation of active functional groups and pores during the activation.In the present study,ACFs with 30 min treatment time in the oxidative process is preferable.

Fig.7 Benzene adsorption capacity of ACFs with different pre-oxidative heating rates

Fig.8 Benzene adsorption capacity of ACFs with different treatment time
3 Conclusions
Pre-oxidative fibers were synthesized from PAN-based fibers using an electrospinning technique and then oxidized underdifferentpre-oxidative conditions.The experiments demonstrate that with the increasing of temperature,heating rate,and treatment time in the oxidative process,more PAN molecules will convert into condensed and thermo-tolerant ladder-shaped structures.Thesestructureswillhinderthe formation ofactivefunctionalgroupsand poresathigh temperature during the activation process,to weaken the benzene adsorption capacity of ACFs.When the pre-oxidative temperature is 230℃ in the present study,the ACFs show the best benzene adsorption capacity.Within the range of heating rate 0.25-1.25 ℃ · min-1in the oxidative process,0.75 ℃·min-1is the optimum parameter for ACFs to have the best benzene adsorption capacity.For the treatment time in the pre-oxidative process,less than 30 min is preferable for ACFs to achieve high benzene adsorption capacity.
[1]Ali A A,El-Hamid M A.Electro-spinning Optimization for Precursor Carbon Nanofibers[J].Composites Part A:Applied Science and Manufacturing,2006,37(10):1681-1687.
[2]Zhang W X.PAN-Based Carbon Fibers[M].Shanghai:Donghua University Press,2005:97-104.(in Chinese)
[3]Yusof N,Ismail A F.Post Spinning and Pyrolysis Processes of Polyacrylonitrile(PAN)-BasedCarbonFiberandActivated Carbon Fiber:a Review[J].Journal of Analytical and Applied Pyrolysis,2012,93:1-13.
[4]Qin X H.Structure and Property of Electrospun PAN Nanofibers by Different Pre-oxidated Heating Rates[J].Journal of Industrial Textiles,2011,41(1):57-69.
[5]Qin X H.Structure and Property ofElectrospinning PAN Nanofibers by Different Preoxidation Temperature[J].Journal of Thermal Analysis and Calorimetry,2010,99(2):571-575.
[6]Esrafilzadeh D,Morshed M,Tavanai H.An Investigation on the Stabilization of Special Polyacrylonitrile Nanofibers as Carbon or Activated Carbon Nanofiber Precursor [J].Synthetic Metals,2009,159(3/4):267-272.
[7]Fazlitdinova A G,Tyumentsev V A,Podkopayev S A,et al.Changes of Polyacrylonitrile Fiber Fine Structure during Thermal Stabilization[J].Journal of Materials Science,2010,45(15):3998-4005.
[8]Hanna S B,Yehia A A,Ismail M N,et al.Preparation and Characterization of Carbon Fibers from Polyacrylonitrile Precursors[J].Journal of Applied Polymer Science,2012,123(4):2074-2083.
[9]GB/T 12496.5—1999.Test Methods of Wooden Activated Carbon:Determination of Carbon Tetrechlodride Activity[S].
[10]Martin S C,Liggat J J,Snape C E.In situ NMR Investigation into the Thermal Degradation and Stabilization of PAN [J].Polymer Degradation and Stability,2001,74(3):407-412.
[11]Dalton S,Heatley F,Budd P M.Thermal Stabilization of Polyacrylonitrile Fibres[J].Polymer,1999,40(20):5531-5543.
[12]Belousova T A.IR Spectroscopic Characteristics of Polyacrylonitrile Copolymer Fibers[J].Fiber Chemistry,2002,34(2):146-150.
[13]Jiang L L,Shi M H,Gao Y M.Experimental Study on Adsorption and Separation Process of Benzene Vapor by Activated Carbon[J].Journal of Engineering Thermophysics,2003,24(2):274-276.
[14]Hsiao H Y,Huang C M,Hsu M Y,et al.Preparation of High-Surface-Area PAN-Based Activated Carbon by Solution-Blowing Process for CO2Adsorption[J].Separation and Purification Technology,2011,82:19-27.
 Journal of Donghua University(English Edition)2012年3期
Journal of Donghua University(English Edition)2012年3期
- Journal of Donghua University(English Edition)的其它文章
- A Novel Preparation of Artificial Bile Ducts for Clinical Application of Biliary Diseases
- Service Robot Localization Based on Global Vision and Stereo Vision
- Synthesis and Characterization of Novel Fluoroalkyl Unsaturated Multi-carboxylic Acid Esters
- Efficient Rate Control Algorithm for Hierarchical Video Coding
- A Modified Differential Evolution for Uniform Parallel Machines Scheduling Problem with Minimizing Makespan
- Sorption Kinetics and Capacity of Composite Materials Made up of Polymeric Fabric and Expanded Perlite for Oil in Water
