Neurotoxicity of intrathecal injections of dexmedetomidine into the rat spinal dorsal horn★
Jiabao Hou, Zhongyuan Xia, Xingpeng Xiao, Xing Wan, Bo Zhao
Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China
Neurotoxicity of intrathecal injections of dexmedetomidine into the rat spinal dorsal horn★
Jiabao Hou, Zhongyuan Xia, Xingpeng Xiao, Xing Wan, Bo Zhao
Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China
To investigate the neurotoxicity of intrathecal injections of dexmedetomidine, Sprague-Dawley rats were intrathecally injected with dexmedetomidine at doses of 0.75, 1.50 and 3.00 μg/kg into the spinal dorsal horn. We found that c-Fos expression in the rat spinal dorsal horn peaked at 7 hours following the 3.00 μg/kg dexmedetomidine injection, while the levels of c-Fos expression following 0.75 and 1.50 μg/kg dexmedetomidine were similar to those in the spinal dorsal horn of normal rats. At 48 hours following administration, the level of c-Fos expression was similar to normal levels. In addition, the intrathecal injections of dexmedetomidine increased paw withdrawal mechanical thresholds and prolonged thermal tail flick latencies. These results indicate that dexmedetomidine has pronounced antinociceptive effects. However, dexmedetomidine appears to have neurotoxic effects in the spinal cord because it increased c-Fos expression in the spinal dorsal horn within 7 hours following administration.
dexmedetomidine; drug toxicity; spinal cord; fos; paw withdrawal mechanical threshold; thermal tail flick latency
Research Highlights
Intrathecal injection of dexmedetomidine at a high dose (3.00 μg/kg) engendered antinociceptive effects and induced c-Fos expression in the spinal dorsal horn of rats.
INTRODUCTION
Dexmedetomidine is a novel and highly selective α2adrenergic receptor agonist, which has eight times higher affinity for α2-adrenergic receptors than clonidine[1].
Dexmedetomidine offers beneficial pharmacological properties and provides dose-dependent sedation, analgesia, sympatholysis, and it does not induce respiratory depression[2-3]. Intrathecal and epidural administration of dexmedetomidine in combination with anesthetic drugs like lidocaine can prolong motor blockade[4-5]. However, epidural administration of dexmedetomidine may have a harmful effect on the myelin sheath. It remains unknown whether intrathecal injections of dexmedetomidine have toxic effects on the spinal cord.
The analgesic effects of dexmedetomidine are achieved through activation of α-2A receptors in the spinal dorsal horn[4,6-7]. Therefore, in the present study, we hypothesized that intrathecal injections of dexmedetomidine may activate α-2A receptors and elicit analgesia. However, whether dexmedetomidine is neurotoxic to the spinal cord remains to be fully elucidated[4,6-7]. c-Fos is the protein of the proto-oncogene c-fos and is expressed after noxious stimuli. Therefore, it has been extensively used as a marker for the neural activation following tissue injuries and nociception in the spinal cord[8-9]. In thepresent study, we assessed the neurotoxicity of intrathecal injections of dexmedetomidine by determining c-Fos expression in the spinal dorsal horn.
RESULTS
Quantitative analysis of experimental animals
A total of 60 Sprague-Dawley rats were equally and randomly assigned to a control group (no intervention), a saline group (intrathecal injection with 10 μL normal saline), or three groups that received different doses of dexmedetomidine (intrathecal injection with 10 μL of 0.75, 1.50, 3.00 μg/kg of dexmedetomidine). All 60 rats were included in the final analysis.
Effects of intrathecal injection of dexmedetomidine on lumbar spinal c-Fos expression
c-Fos expression was induced in the neuronal nuclei of the lumbar spinal cord. It was primarily distributed to the dorsal horn of gray matter and central canal, especially in the lamina I and II, and also in the other laminas of gray matter. In contrast, c-Fos was scarcely expressed in the white matter (Figure 1).
The density of the positive neuronal nuclei in the dorsal horn, where c-Fos was primarily expressed, was analyzed (× 100). c-Fos expression ranged from little to none in the spinal cord of the control rats. 7 or 24 hours after intrathecal injections of dexmedetomidine the level of c-Fos expression was the highest in the animals that received 3.00 μg/kg of dexmedetomidine (P< 0.05). In contrast, c-Fos levels were not significantly different in the animals in the other four groups. However, no significant differences were observed among groups 48 hours after the dexmedetomidine administrations (Table 1).
Effects of intrathecal injection of dexmedetomidine on rat behaviors
Paw withdrawal mechanical threshold (PWMT)
Baseline measures of the PWMT in left hind paw were similar among all of the groups. Compared with baseline measurements, the PWMTs of the subjects in each group rose significantly 30 minutes after intrathecal injections of dexmedetomidine (P< 0.05), and they were significantly higher than the control and saline-treated subjects (P<0.05).
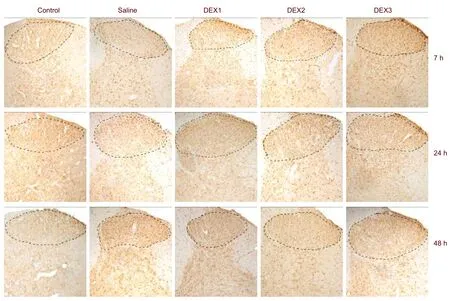
Figure 1 c-Fos expression in the dorsal horn of animals from each group (immunohistochemical staining, × 100).
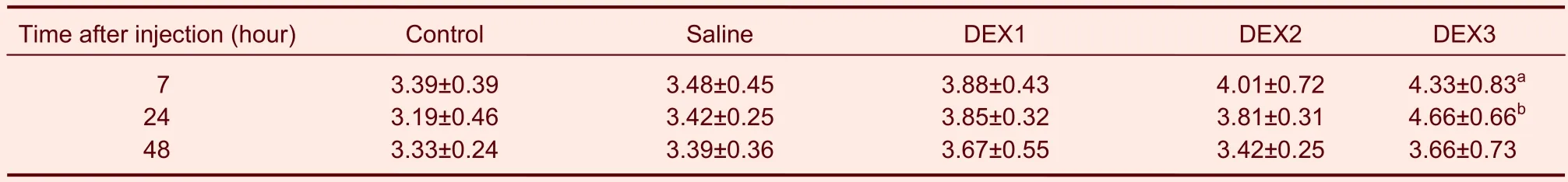
Table 1 Influence of DEX on c-Fos expression (100-fold field of view) in the dorsal horn of rats
Moreover, the PWMTs were greater in the animals that received 3.00 μg/kg of dexmedetomidine compared with those that received the other doses of dexmedetomidine (P< 0.05). There were no significant differences between the animals that received the lower doses of dexmedetomidine (Table 2).
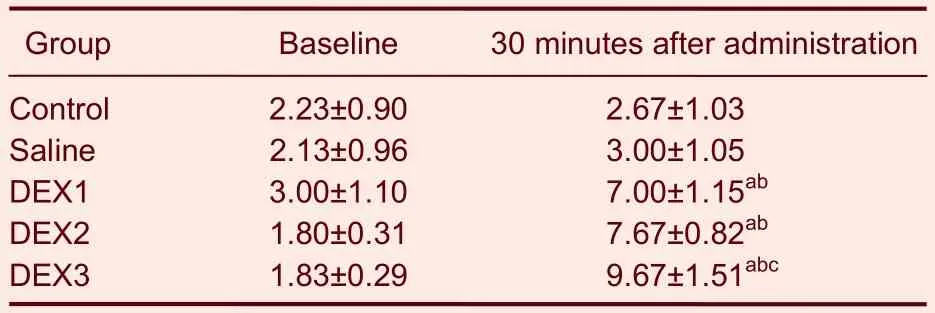
Table 2 Effects of intrathecal injections of DEX on paw withdrawal mechanical thresholds (g)
Thermal tail flick latency (TFL)
Baseline TFLs were not significantly different among the animals in the different groups. Sixty minutes after intrathecal injection of dexmedetomidine, the latencies of the animals that received each dose of dexmedetomidine were longer than at baseline (P <0.05) and longer than the latencies in the control and saline-treated animals (P <0.05). In particular, TFLs were the longest in the animals that received 3.00 μg/kg of dexmedetomidine (Table 3).
DISCUSSION
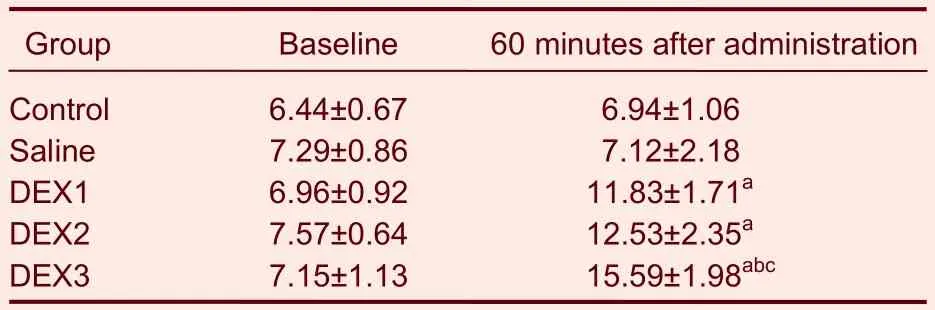
Table 3 Effects of intrathecal injections of DEX on thermal tail flick latencies (second)
Intrathecal administration of dexmedetomidine can inhibit C fiber-evoked responses and slow ventral root potentials in spinal dorsal horn neurons[10]. Dexmedetomidine exerts spinal antinociception[11]by releasing norepinephrine. This released norepinephrine acts on the α-2A adrenoceptors in the presynaptic and postsynaptic membrane to increase norepinephrine levels in the cerebrospinal fluid, induce acetylcholine release, and elicit synthesis and release of nitrous oxide[12-13]. Generally, the antinociceptive effects of intrathecal administration of dexmedetomidine were dose-dependent[14-15]. The strongest analgesic effects were engendered by the 3.00 μg/kg dose of dexmedetomidine, whereas there were no significant differences between the lower doses.
Since α-2A adrenoceptors are mainly in the postsynaptic membrane of the dorsal horn[16], and c-Fos is mainly expressed in the dorsal horn of spinal cord, it is expressed only in nuclei. c-Fos is sparsely expressed under normal conditions, but its expression is increased when there are insults to the spinal cord. Moreover, the level of c-Fos expression is positively correlated with the magnitude of the insult[17]. Thus, it is believed to be a valid index of neurotoxicity. Hayashiet al[18]found that the rate, frequency, and magnitude of c-fos expression depended on different stimulation factors. A previous study showed that c-Fos protein expression peaks between 7 and 24 hours after intrathecal injections. Therefore, in the present study, we selected 7, 24 and 48 hours as the observation time. Our findings suggest that dexmedetomidine had antinociceptive effects and induceed c-Fos expression in the dorsal horn. Moreover, the peak dose and time point of these effects were 3.00 μg/kg and between 7 and 24 hours, respectively. This suggests that lower doses of dexmedetomidine did not harm the spinal cord, whereas 3.00 μg/kg of dexmedetomidine did harm the spinal cord.
In summary, intrathecal injections of dexmedetomidine at low doses (0.75 and 1.50 μg/kg) can relieve pain without engendering neurotoxicity, whereas a large dose of dexmedetomidine (3.00 μg/kg) can induce strong anti-nociceptive effects but significantly increased c-Fos expression in the dorsal horn within 7 hours of administration.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Laboratory of Anesthesiology, Renmin Hospital of Wuhan University, China from November 2009 to July 2010.
Materials
A total of 60 male, specific pathogen-free Sprague-Dawley rats, weighing 180-220 g, were provided by the Institute of Laboratory Animal Science, Tongji Medical College, Huazhong University of Science and Technology, China (license No. SCXK (E) 2004- 0007). All rats were housed under constant temperature (20 ± 2°C) and with 12-hour light/dark cycles. They were allowed free access to water and food. The animal protocol complied with theGuidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China in 2006[19].
Methods
Catheter placement
Animals were intraperitoneally anesthetized using 300 mg/kg chloral hydrate. The hair on their waist was shaved and scrubbed with 10% povidone iodine. A 2-cm longitudinal incision was made along the lumbar vertebrae. Connective tissues and the paraspinal muscles were dissected, and the L5-6lumbar gap was found. A 23G needle was used to cut out the gap and a PE10 tube (0.25 mm ID, polyeurethane, 10 cm; AniLab software and instruments Co. Ltd, Ningbo, China) was inserted. The tube was snugly slipped into the gap measuring about 4 cm, and then the free edge of the catheter was placed in the subcutaneous tunnel and blocked by fire[20-21](supplementary Figure 1 online). The catheter was subsequently fixed to the skin, and the incision was sutured. 10 μL of 2% lidocaine was intrathecally injected 72 hours later. We accepted that the catheter was correctly placed if the rat lost sensory and motor function after a lidocaine administration[21].
Dexmedetomidine administration
Three days after the catheter placement, the animals in the control group received no intervention, and the animals in the saline group and dexmedetomidine groups received intrathecal injections with 10 μL normal saline or 0.75, 1.50, or 3.00 μg/kg of dexmedetomidine (Hengrei Co. Ltd., Lianyungang, China).
Behavioral test
PWMT: The PWMT was determined both before (baseline) and 30 minutes after drug administration. Before the tests, the rats were placed in a clear PlantarVon FreyTMplexiglass box (Stoelting, WoodDale, IL, USA) with a wire mesh floor. Twelve rats were tested in each group. After approximately 20 minutes of habituation, an ascending series of mechanical stimuli of von Frey filaments with logarithmically incremental stiffness (1.4, 2.0, 4.0, 8.0, 10.0, 12.0 and 15.0 g) were applied to the plantar surface of the left hind paw[22]. A positive response was defined as withdrawal from the Von Frey filament. Confirmation of the threshold was tested by examining the filament above and below the withdrawal response[20,22-23]. Each rat was tested three times with an interval of 5 minutes between each application. A decrease in the mechanical withdrawal threshold was interpreted as mechanical hyperalgesia[20].
TFL: The test was conducted in a quiet, dimly lit room with an ambient temperature of 20 ± 2°C. The rats were marked with a pen on the tail about 1/3 of the length from the tip. A light beam was focused on this marked site.
The radiant heat tail flick latency was tested at baseline and 60 minutes after the intrathecal injections. Each rat was tested three times with an interval of 5 minutes between each application. The average of these three tests was used to calculate the TFL[24-26]. To prevent tissue damage, the exposure time was limited to less than 20 seconds.
Sample preparation
Four rats in each group were intraperitoneally anesthetized using 300 mg/kg chloral hydrate 7, 24, or 48 hours after the behavioral tests. Then, transcardial perfusion was applied with a 4% paraformaldehyde solution (pH 7.6; 500 mL). The entire spinal cord in each rat was removed from its spinal column and fixed overnight using a 4% paraformaldehyde solution at 4°C. After fixation, the spinal cords were cryoprotected in 30% sucrose for a minimum of 24 hours[4]. A series of 25 μm coronal sections from these lumbar spinal cords were cut with a freezing microtome (Leica, Germany). Free-floating sections from each rat were stored in cold 0.1 M PBS and then processed to detect c-Fos protein expression.
Immunohistochemistry for density of Fos-positive cells
Immunohistochemical staining was performed using the strept-avidin-biotin peroxidase method. Sections were incubated in 3% H2O2solution for 15 minutes and rinsed twice in 0.1 M PBS. Then they were incubated in 5% BSA confining liquid for 30 minutes at room temperature, incubated into a polyclonal rabbit anti-c-Fos (Boster Biotechnology Co., Ltd., Wuhan, China) at a 1:50 dilution overnight at 4°C, washed three times in PBS for 5 minutes each, placed into biotinylated goat anti-rabbit IgG (ready-to-use kit; Boster Biotechnology Co., Ltd.), and incubated for 2 hours at 20°C. Afterwards, they were again washed three times in PBS for 5 minutes each and placed into a strept-avidin-biotin complex (ready-to-use kit; Boster Biotechnology Co., Ltd.) for an hour at 20°C. Following three PBS rinses, they were incubated in a 0.05% 3,3’-diaminobenzidine solution until the desired levels of staining were reached. The reaction was terminated with a PBS rinse. The positively stained nuclei could be clearly seen under microscopic visualization at 400 × magnification. Sections were then mounted onto the slides with a gelatine solution. They were then left to air dry at room temperature before being dehydrated through serial applications starting with alcohol and proceeding to histolene. They were then placed under a coverslip with DPX[4,22,27]. Five sections were randomly selected from each rat, analyzed with a microscope (Olympus, Tokyo, Japan), and photographed with a digital camera. The images were analyzed using Image Pro Plus 6.0. The density of positive c-Fos-like immunoreactive cells was quantified (points/area).
Statistical analysis
Statistical analyses were performed using GraphPad prism 5.0 (GraphPad Software, San Diego, CA, USA). The data are expressed as mean ± SD, with the exception of the TFL data, which were calculated as the average of three replicates and are expressed as mean ± SEM. Differences were evaluated using a two-way analysis of variance, followed by a Student-Newman-Keuls test.Pvalues of less than 0.05 were considered to be statistically significant.
Author contributions: Jiabao Hou designed this study, wrote the manuscript and processed the experimental data. Zhongyuan Xia guided the experiment and revised the manuscript. Xing Wan translated and corrected this manuscript. Xingpeng Xiao designed the study and analyzed the data. Bo Zhao contributed to the statistical analysis.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Ethics Committee of Wuhan University, China.
Supplementary information: Supplementary data associated with this article can be found by visiting www.nrronline.org.
[1] Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28(1):3-6.
[2] Hosokawa K, Shime N, Kato Y, et al. Dexmedetomidine sedation in children after cardiac surgery. Pediatr Crit Care Med. 2010;11(1):39-43.
[3] Su F, Hammer GB. Dexmedetomidine: pediatric pharmacology, clinical uses and safety. Expert Opin Drug Saf. 2011;10(1):55-66.
[4] Konakci S, Adanir T, Yilmaz G, et al. The efficacy and neurotoxicity of dexmedetomidine administered via the epidural route. Eur J Anaesthesiol. 2008;25(5):403-409.
[5] Calasans-Maia JA, Zapata-Sudo G, Sudo RT. Dexmedetomidine prolongs spinal anaesthesia induced by levobupivacaine 0.5% in guinea-pigs. J Pharm Pharmacol. 2005;57(11):1415-1420.
[6] Kanazi GE, Aouad MT, Jabbour-Khoury SI, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50(2): 222-227.
[7] Fairbanks CA, Kitto KF, Nguyen HO, et al. Clonidine and dexmedetomidine produce antinociceptive synergy in mouse spinal cord. Anesthesiology. 2009;110(3):638-647.
[8] Gao YJ, Ji RR. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009;2:11-17.
[9] Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987; 328(6131):632-634.
[10] Okamoto T, Ishifuji S, Taoda M, et al. Dexmedetomidine for sedation during voice monitoring surgery in a patient with an episode of seizure. Masui. 2008;57(8):1013-1016.
[11] Kaya FN, Yavascaoglu B, Turker G, et al. Intravenous dexmedetomidine, but not midazolam, prolongs bupivacaine spinal anesthesia. Can J Anaesth. 2010;57(1):39-45.
[12] Dawson C, Ma D, Chow A, et al. Dexmedetomidine enhances analgesic action of nitrous oxide: mechanisms of action. Anesthesiology. 2004;100(4):894-904.
[13] Tamagaki S, Suzuki T, Hagihira S, et al. Systemic daily morphine enhances the analgesic effect of intrathecal dexmedetomidine via up-regulation of alpha 2 adrenergic receptor subtypes A, B and C in dorsal root ganglion and dorsal horn. J Pharm Pharmacol. 2010;62(12):1760-1767.
[14] Fisher B, Zornow MH, Yaksh TL, et al. Antinociceptive properties of intrathecal dexmedetomidine in rats. Eur J Pharmacol. 1991; 192(2):221-225.
[15] Ayoglu H, Gul S, Hanci V, et al. The effects of dexmedetomidine dosage on cerebral vasospasm in a rat subarachnoid haemorrhage model. J Clin Neurosci. 2010;17(6):770-773.
[16] van Oostrom H, Stienen PJ, Doornenbal A, et al. The alpha(2)-adrenoceptor agonist dexmedetomidine suppresses memory formation only at doses attenuating the perception of sensory input. Eur J Pharmacol. 2010;629(1-3):58-62.
[17] Yan M, Zhang LC, Dai TJ, et al. Propofol depresses c-fos expression of NOS neurons in the spinal cord of rats with inflammatory pain. Sheng Li Xue Bao. 2002;54(1):60-64.
[18] Hayashi M, Ueyama T, Nemoto K, et al. Sequential mRNA expression for immediate early genes, cytokines, and neurotrophins in spinal cord injury. J Neurotrauma. 2000;17(3): 203-218.
[19] The Ministry of Science and Technology of the People’s Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006-09-30.
[20] Sluka KA, Audette KM. Activation of protein kinase C in the spinal cord produces mechanical hyperalgesia by activating glutamate receptors, but does not mediate chronic muscle-induced hyperalgesia. Mol Pain. 2006;2:13.
[21] Milligan ED, Hinde JL, Mehmert KK, et al. A method for increasing the viability of the external portion of lumbar catheters placed in the spinal subarachnoid space of rats. J Neurosci Methods. 1999; 90(1):81-86.
[22] Cao F, Gao F, Xu AJ, et al. Regulation of spinal neuroimmune responses by prolonged morphine treatment in a rat model of cancer induced bone pain. Brain Res. 2010;1326:162-173.
[23] Gopalkrishnan P, Sluka KA. Effect of varying frequency, intensity, and pulse duration of transcutaneous electrical nerve stimulation on primary hyperalgesia in inflamed rats. Arch Phys Med Rehabil. 2000;81(7):984-990.
[24] Dogrul A, Gülmez SE, Deveci MS, et al. The local antinociceptive actions of nonsteroidal antiinflammatory drugs in the mouse radiant heat tail-flick test. Anesth Analg. 2007;104(4):927-935.
[25] Le Bars D, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53(4):597-652.
[26] Dagci T, Sengul G, Keser A, et al. NADPH-d and Fos reactivity in the rat spinal cord following experimental spinal cord injury and embryonic neural stem cell transplantation. Life Sci. 2011; 88(17-18):746-752.
[27] Allbutt HN, Siddall PJ, Keay KA. Contusive spinal cord injury evokes localized changes in NADPH-d activity but extensive changes in Fos-like immunoreactivity in the rat. J Anat. 2007; 211(3):352-370.
Cite this article as:Neural Regen Res. 2012;7(23):1765-1770.
Jiabao Hou★, Master, Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China
Zhongyuan Xia, Ph.D., Professor, Department of Anesthesiology, Renmin Hospital of Wuhan University, Wuhan 430060, Hubei Province, China
xiazhongyan2005@yahoo. com.cn
2011-11-22
2012-03-06
(N20110416001/YJ)
Hou JB, Xia ZY, Xiao XP, Wan X, Zhao B. Neurotoxicity of intrathecal injections of dexmedetomidine into the rat spinal dorsal horn. Neural
Regen Res. 2012;7(23):1765-1770.
www.crter.cn
www.nrronline.org
10.3969/j.issn.1673-5374. 2012.23.001
We thank the Laboratory of Anesthesiology, Tongji Hospital, Huazhong University of Science and Technology in China for their support with equipment.
(Edited by Gao SY, Jia DL/Su LL/Song LP)
- 中国神经再生研究(英文版)的其它文章
- Survey of spinal cord injury-induced neurogenic bladder studies using the Web of Science
- Current therapeutic strategies for inflammation following traumatic spinal cord injury☆●
- Stem cell therapy in neurodegenerative diseases From principles to practice●
- Meta-analysis of efficacy of topiramate in migraine prophylaxis★
- Differentiation of endogenous neural stem cells in adult versus neonatal rats after brachial plexus root avulsion injury*☆
- An enriched environment improves cognitive performance in mice from the senescenceaccelerated prone mouse 8 strain Role of upregulated neurotrophic factor expression in the hippocampus***☆

