Acupuncture inhibits cue-induced heroin craving and brain activation**★
Xinghui Cai, Xiaoge Song, Chuanfu Li, Chunsheng Xu, Xiliang Li, Qi Lu
1 School of Acupuncture and Orthopedics, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
2 Institute of Acupuncture and Meridians, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
3 Medical Imaging Center, First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei 230031, Anhui Province, China
Acupuncture inhibits cue-induced heroin craving and brain activation**★
Xinghui Cai1, Xiaoge Song2, Chuanfu Li3, Chunsheng Xu3, Xiliang Li2, Qi Lu3
1School of Acupuncture and Orthopedics, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
2Institute of Acupuncture and Meridians, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
3Medical Imaging Center, First Affiliated Hospital of Anhui University of Traditional Chinese Medicine, Hefei 230031, Anhui Province, China
Previous research using functional MRI has shown that specific brain regions associated with drug dependence and cue-elicited heroin craving are activated by environmental cues. Craving is an important trigger of heroin relapse, and acupuncture may inhibit craving. In this study, we performed functional MRI in heroin addicts and control subjects. We compared differences in brain activation between the two groups during heroin cue exposure, heroin cue exposure plus acupuncture at theZusanlipoint (ST36) without twirling of the needle, and heroin cue exposure plus acupuncture at theZusanlipoint with twirling of the needle. Heroin cue exposure elicited significant activation in craving-related brain regions mainly in the frontal lobes and callosal gyri. Acupuncture without twirling did not significantly affect the range of brain activation induced by heroin cue exposure, but significantly changed the extent of the activation in the heroin addicts group. Acupuncture at theZusanlipoint with twirling of the needle significantly decreased both the range and extent of activation induced by heroin cue exposure compared with heroin cue exposure plus acupuncture without twirling of the needle. These experimental findings indicate that presentation of heroin cues can induce activation in craving-related brain regions, which are involved in reward, learning and memory, cognition and emotion. Acupuncture at theZusanlipoint can rapidly suppress the activation of specific brain regions related to craving, supporting its potential as an intervention for drug craving.
acupuncture;Zusanli(ST36); heroin addiction; cues induction; functional MRI; craving; twirling; activation of brain regions; traditional Chinese medicine; neural regeneration
Research Highlights
(1) Because there have been no previous controlled comparisons, in this study, to provide objective and convincing results, brain activation was compared under heroin cue exposure, heroin cue exposure plus acupuncture at theZusanlipoint (ST36) without twirling of the needle, and heroin cue exposure plus acupuncture at theZusanlipoint with twirling of the needle.
(2) Heroin cues exposure activated craving-related brain regions such as the frontal lobe, which have been associated with reward, learning and memory, cognition, and emotion.
(3) Because acupuncture at theZusanlipoint with twirling of the needle rapidly suppressed the activation of the craving-related brain regions, it may be a viable intervention for drug craving.
Abbreviation
fMRI: functional MRI
INTRODUCTlON
In the present study, we sought to understand whether drug addicts and non-addicts exhibit the same heroin craving during environmental cue exposure, whether the distribution of activated brain regions is related to craving during such exposure, and whether acupuncture influences this brain activation in drug addicts. We examined these processes by investigating the influence of acupuncture on the brain activation of heroin addicts during environmental cue exposure, in a broader attempt to provide a basis for craving suppression. Environmental cues can activate brain regions associated with learning and memory and reward in heroin addicts, and functional MRI (fMRI) can reveal these craving-related brain regions[1]. However, the influence of acupuncture on cue-elicited brain activation in heroin addicts remains unclear. Previous studies have found that heroin cue exposure may induce the activation of craving-related brain regions, which are involved in reward, learning and memory, cognition and emotion. Moreover, acupuncture at theZusanlipoint (ST36) can rapidly suppress activity in these craving-related brain regions. However, these experimental results are limited by small sample sizes, lack of comparisons with subjects not addicted to heroin, and the two-dimensional images often provided by brain fMRI. As such, previous studies have not convincingly demonstrated that acupuncture is an effective intervention for craving.
Current research methods to study the influence of acupuncture on cue-elicited brain activation include: (1) comparisons of brain fMRI between heroin addicted and control subjects during heroin cue exposure, (2) comparisons of right-left, anterior-posterior, and inferior-superior shift values among the Brodmann areas activated by heroin cue exposure, and (3) threedimensional enhancement of brain fMRI detection results.
In this study, we aimed to understand the influence of heroin cue exposure on brain activation in heroin addicted and control subjects, investigate acupuncture at theZusanlipoint as an intervention for heroin craving, and determine the neurobiological mechanisms by which acupuncture reduces heroin craving.
RESULTS
Quantitative analysis of participants
Twelve heroin addicts were included in the heroin addicts group, and another 12 male adults with no opioid or other psychoactive drug dependence were included in the control group. The two groups were well matched in terms of age and gender (Table 1).
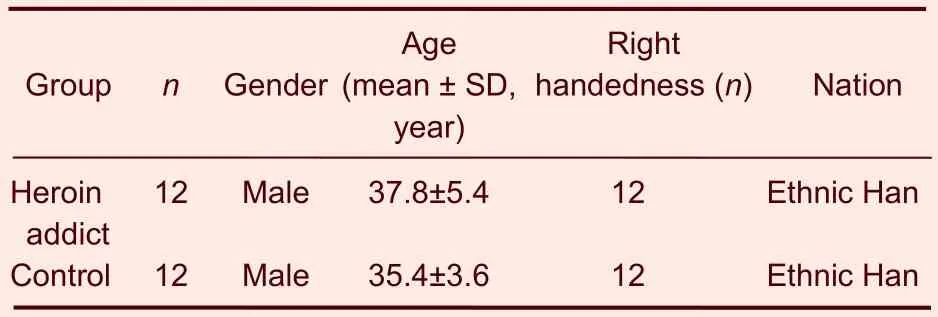
Table 1 Baseline characteristics of the participants in the two groups
One subject in the heroin addicts group was excluded because his head motion range exceeded 2 mm or 2° during the fMRI scanning (Figure 1). The experimental data in the remaining 23 participants were included in the analyses, including 11 subjects in the heroin addicts group and 12 subjects in the control group.
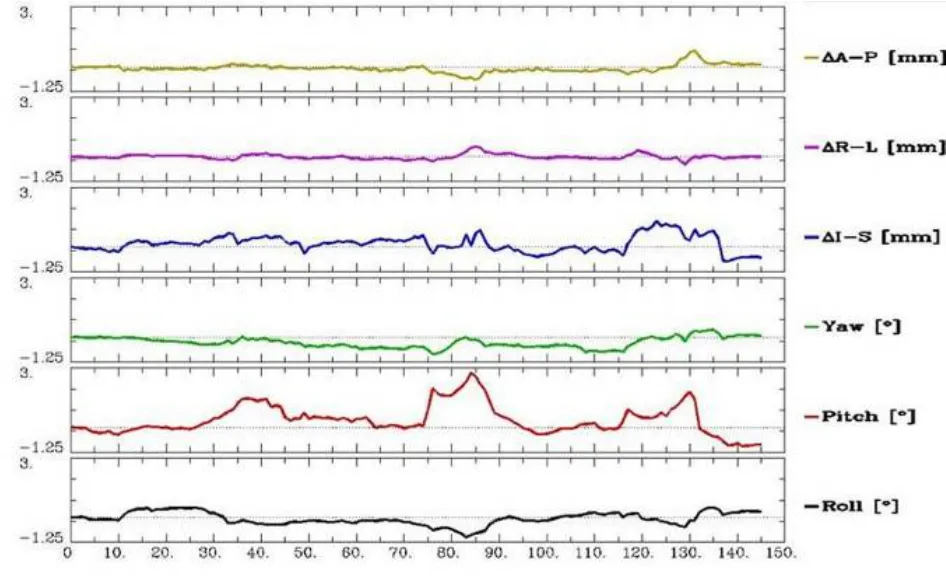
Figure 1 Adjusted parametric curve of head movement during the functional MRI scanning (>2 mm).
Comparison of brain activation induced by heroin cues
In the heroin addicts group, heroin cue exposure activated on average 26 brain regions, which were mainly located in the frontal lobe, parietal lobe, temporal lobe, insula, callosal gyrus, and cerebella (Table 2). In contrast, in the control group, heroin cue exposure activated 5 brain regions, mainly at the gyri centrales and insula (Table 3). This is evidence that heroin cue exposure can activate more and a wider distribution of brain regions in the heroin addicts group than that in the control group (Figures 2, 3). The activated brain regions were the frontal lobe (left medial frontal gyrus, left and right midfrontal gyrus, left and right paracentral lobule, right medial frontal gyrus), left precuneus, temporal lobe (left and right superior temporal gyrus, left and right middle temporal gyrus), parietal lobe (right postcentral gyrus, right supramarginal gyrus), limbic lobe (left cingulate, left and right posterior cingulate), left and right insular lobe, left and right lenticular nucleus, and the cerebellum (semilunar lobule in left cerebellum, left cauda cerebelli). These regions are all related to the cue-induced craving.
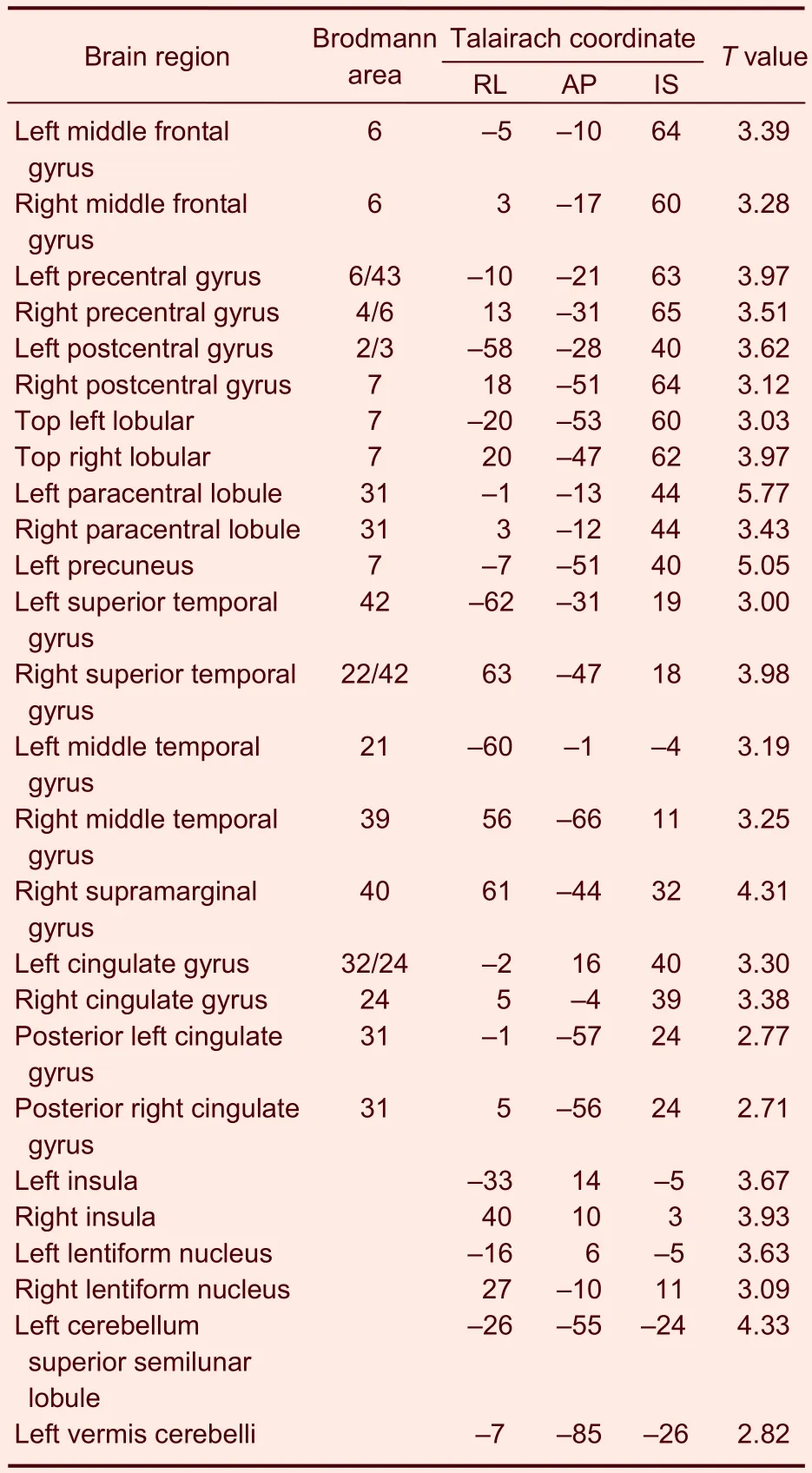
Table 2 Activated brain regions in the heroin addicts group during heroin cue exposure
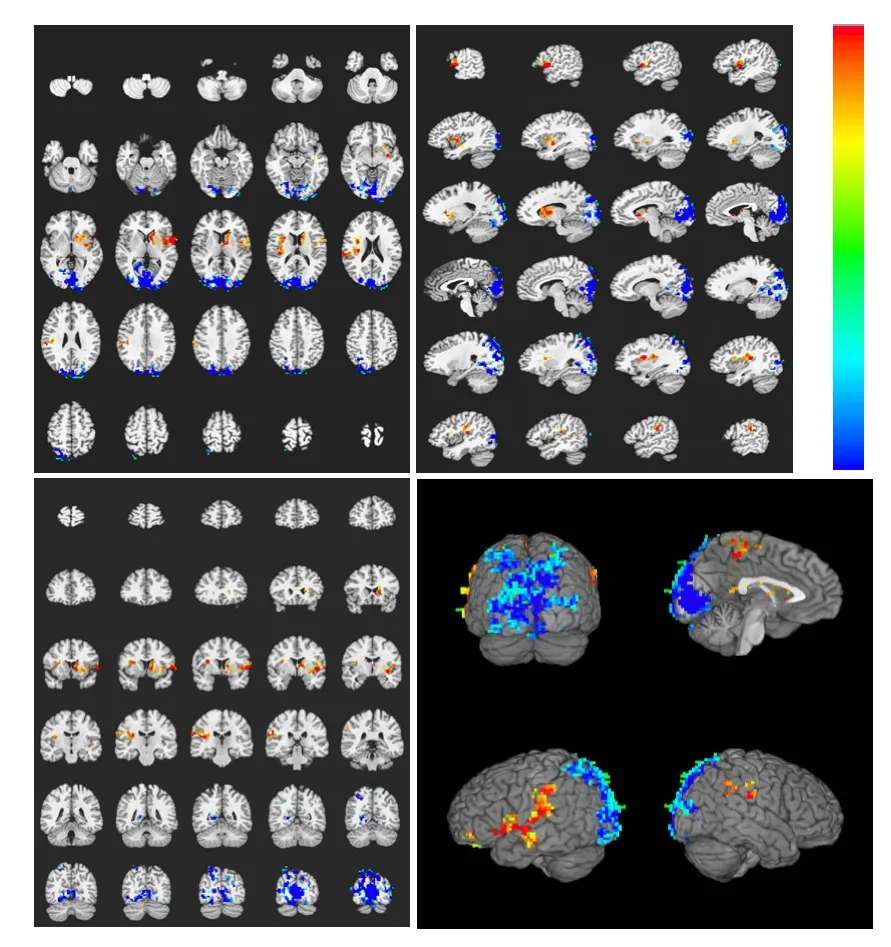
Figure 2 Brain activation profile in the control group during heroin cue exposure.
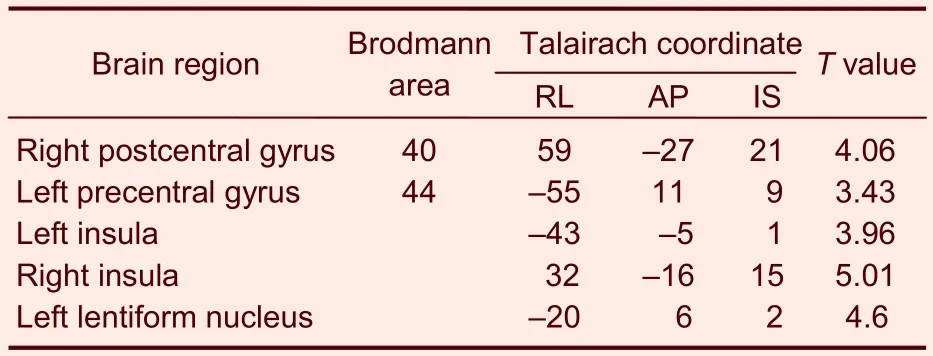
Table 3 Activated brain regions in the control group during heroin cue exposure
Comparison of brain activation during heroin cue exposure plus acupuncture at the Zusanli point without twirling
In the heroin addicts group, under heroin cue exposure and acupuncture at theZusanlipoint without twirling of the needle, there were 27 activated brain regions on average. These areas were mainly located in the frontal lobe, parietal lobe, temporal lobe, callosal gyrus, insula, and cerebella (Figure 4, Table 4).
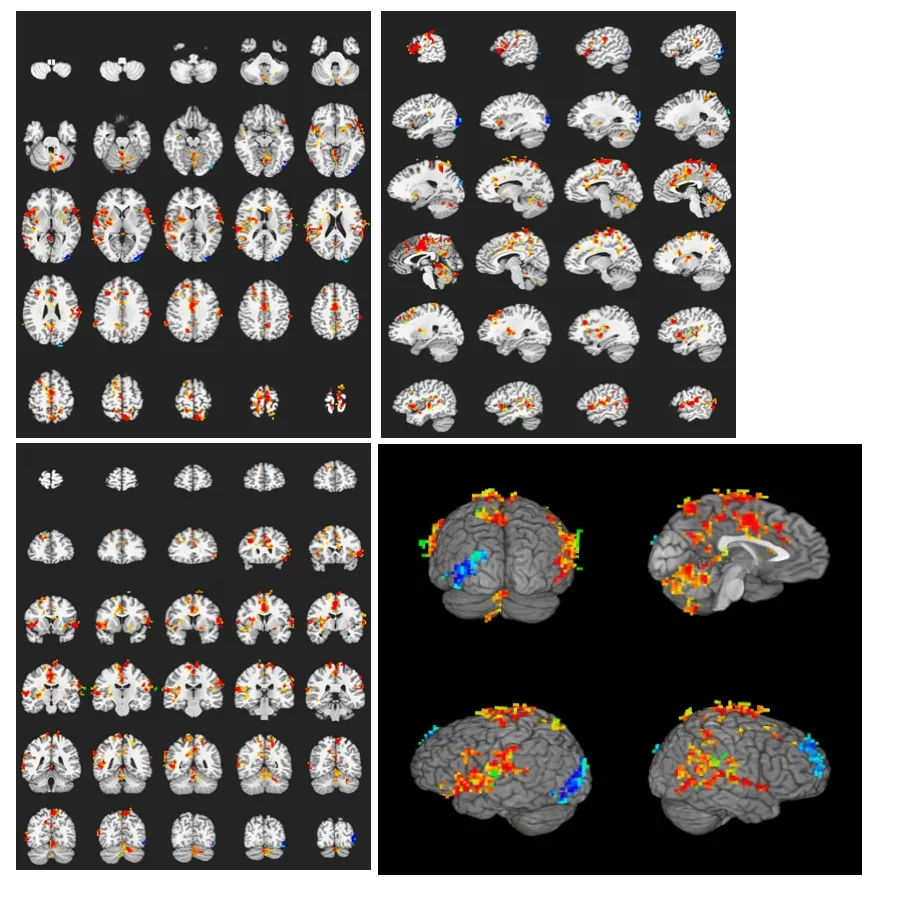
Figure 3 Brain activation profile in the heroin addicts group during heroin cue exposure.
In the control group, under the same conditions, there were seven activated brain regions on average, mainly located at the gyri centrales, parietal lobe, insula callosal, right cingulate gyrus, and cerebella (Table 5, Figure 5). These experimental findings indicate that heroin cue exposure and acupuncture at theZusanlipoint without twirling of the needle did not significantly affect the range of brain activation as compared with heroin cue exposure alone, but significantly changed the extent of the activation in the heroin addicts group.
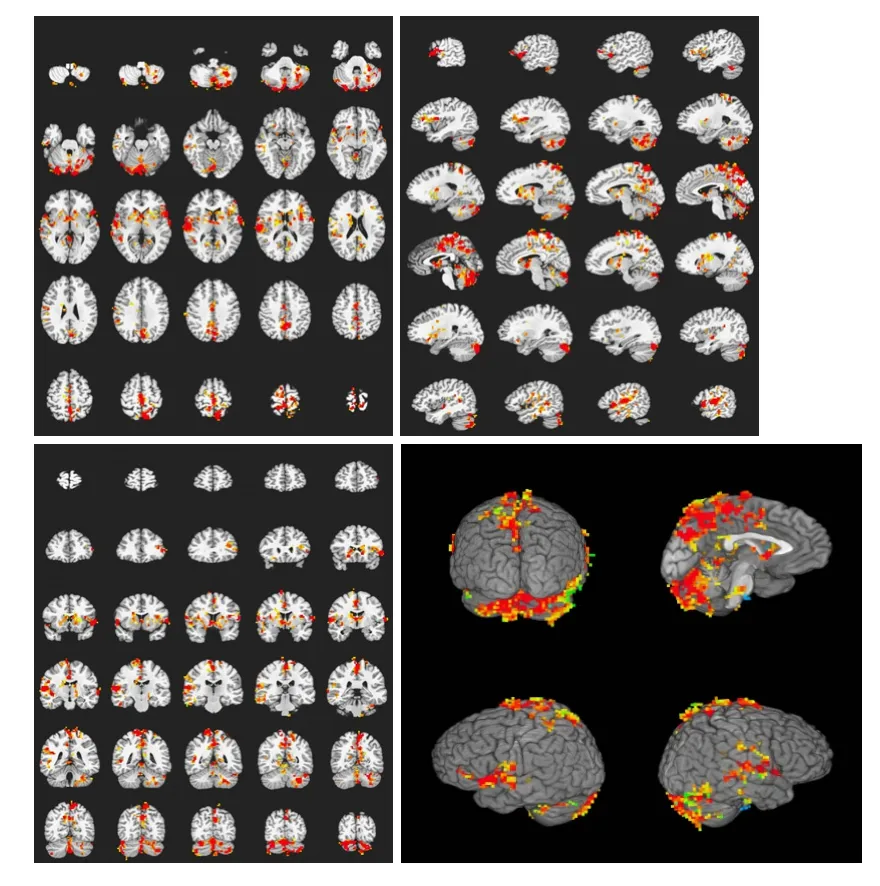
Figure 4 Brain activation profile in the heroin addicts group during heroin cue exposure and acupuncture at the Zusanli point (ST36) without twirling of the needle.
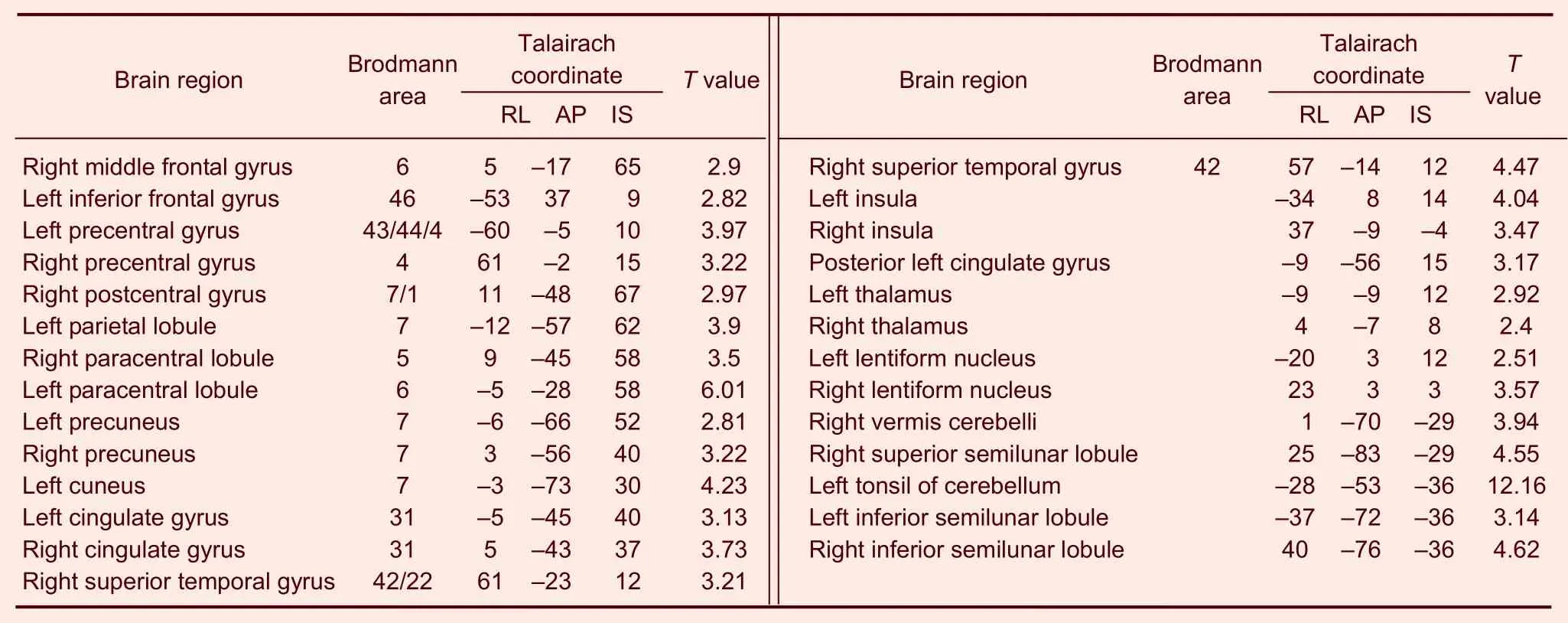
Table 4 Activated brain regions of the heroin addicts group under heroin cue exposure plus acupuncture at Zusanli (ST36) (non-twirling)

Table 5 Activated brain regions in the control group during heroin cue exposure and acupuncture at the Zusanli point (ST36) without twirling of the needle
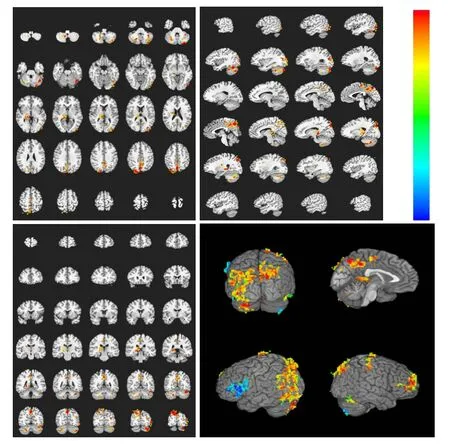
Figure 5 Brain activation profile in the control group during heroin cue exposure and acupuncture at the Zusanli point (ST36) without twirling of the needle.
Comparison of brain activation during heroin cue exposure plus acupuncture at the Zusanli point with twirling of the needle
In the heroin addicts group, under heroin cue exposure and acupuncture at theZusanlipoint with twirling of the needle, there were 12 activated brain regions on average, mainly distributed to the occipital lobe, cuneate lobe, lingual gyrus, and cerebella (Table 6). In the control group, under heroin cue exposure and acupuncture at theZusanlipoint with twirling of the needle, there were eight activated brain regions on average, mainly distributed to the left middle frontal gyrus, right precuneus, left middle occipital gyrus, right thalamus, and cerebellum (Table 7).
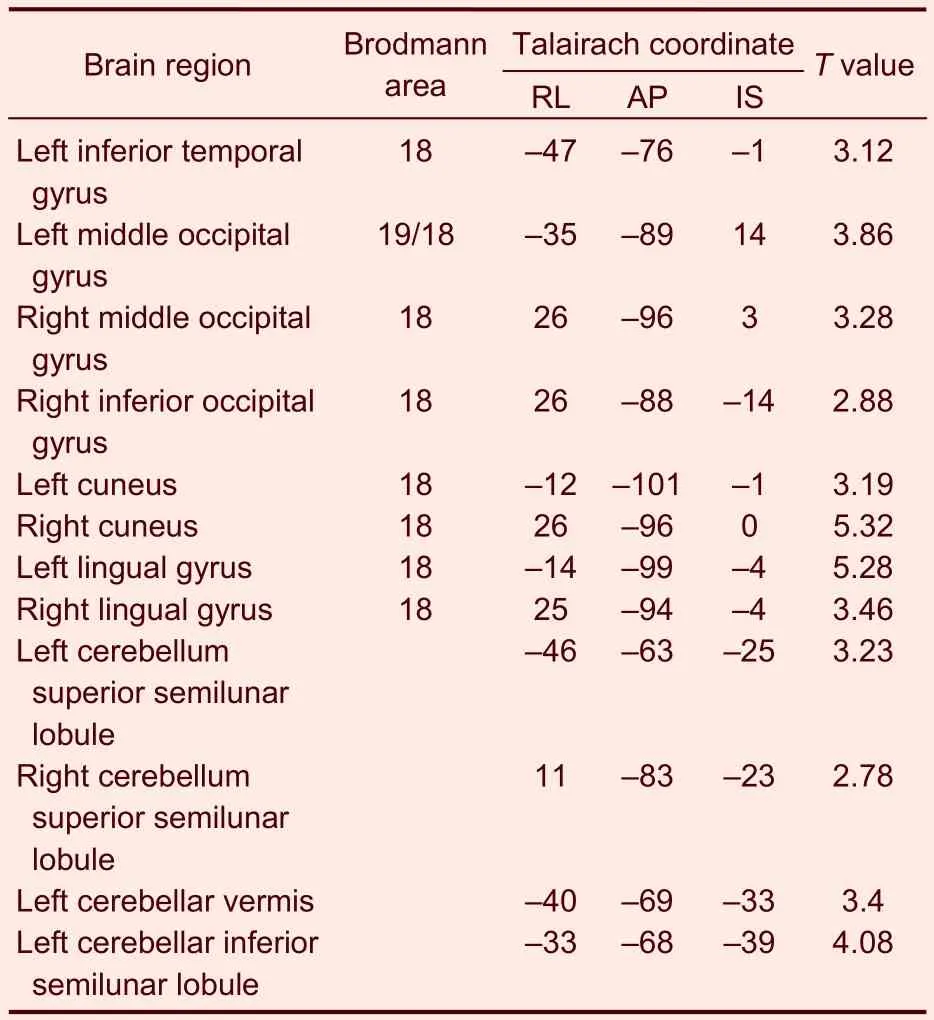
Table 6 Activated brain regions in the heroin addicts group during heroin cue exposure and acupuncture at the Zusanli point (ST36) with twirling of the needle
These experimental findings indicate that heroin cue exposure and acupuncture at theZusanlipoint with twirling of the needle significantly diminished both the range and extent of brain activation, and suppressed activity in the cingulate gyrus and insular lobe. In contrast, the activations of these brain regions were not significantly different in the control group (Figures 6, 7).
DISCUSSION
It has been reported that environmental cues can induce craving[3-6]. Craving is an intense desire for and emotional response to addictive drug effects.
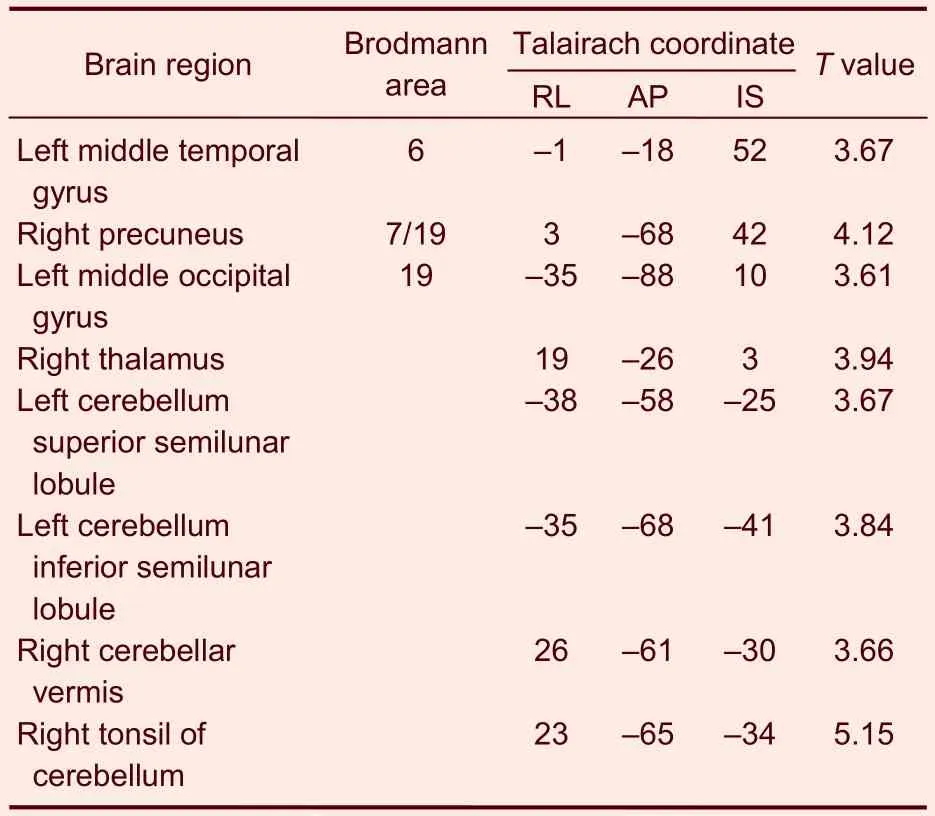
Table 7 Activated brain regions in the control group during heroin cue exposure and acupuncture at the Zusanli point (ST36) with twirling of the needle
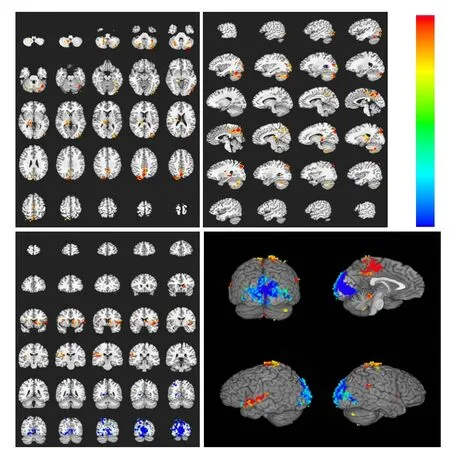
Figure 6 Brain activation profile in the control group during heroin cue exposure and acupuncture at the Zusanli point (ST36) with twirling of the needle.
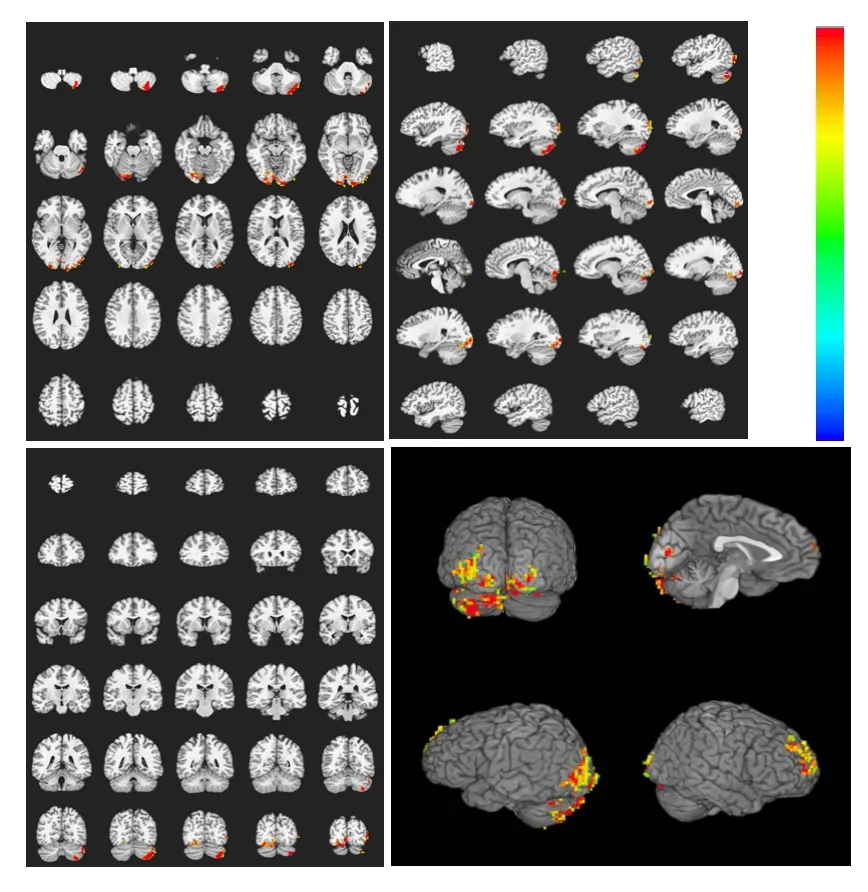
Figure 7 Brain activation profile in the heroin addicts group during heroin cue exposure and acupuncture at the Zusanli point (ST36) with twirling of the needle.
It may be triggered by seeing objects or experiencing situations that are associated with drug use, and often result in relapse or reinstatement of drug seeking. Our present study showed that heroin cues activated more and a wider distribution of brain regions in heroin addicts compared with control subjects. These activations in the frontal lobe, precuneus, temporal lobe, parietal lobe, cingulate gyrus, left and right lenticular nucleus, and cerebellum were associated with cue-induced craving. Significant activation of cortex in their frontal lobe suggests increased attention of the addicts to seeking heroin cues and the engagement of working memory[7-9]. The parietal lobe plays a leading role in spatial feature information processing[10], and the temporal lobe is responsible for some aspects of cognitive functions[11-14]. The cingulate gyrus is specifically activated under the emotional state of drug craving, and has a unique role in craving elicited by environmental cues. The cerebellum has a role in emotional mediation and cognitive processes that is of increasing concern[15], and the precuneus is involved in self-related information processing and self-awareness of cognitive functions. According to the neurophysiological mechanisms of the fMRI signal, when addicts pay more attention to drug cues, increased neural activity in craving-related brain regions will cause increased demand for oxygen and increased blood flow in that area. This is called activation. Our present results confirm that heroin cues can activate craving-related brain regions, which is consistent with previous reports.
TheZusanlipoint can produceQiofSan Jiaoand it is one of the most important points to nourish vigor, regulateqiand blood, and tonify deficiencies. It is especially effective in the treatment of chronic asthenic diseases, such as those of the immune, nervous, and endocrine systems. Recent studies using fMRI have examined the activation of brain regions after acupuncture at theZusanlipoint[16-18]. Acupuncture at theZusanlipoint was shown to cause the functional changes in the prefrontal and temporal lobes[19]. These fMRI results showed that brain blood of the ipsilateral hypothalamus, paraventricular nucleus, and bilateral temporal lobe increased with acupuncture at theZusanlipoint[20]. An increasing number of studies[21-23]have found an influence of acupuncture on craving in drug addicts. Acupuncture can control withdrawal symptoms and improve the patient’s mood, and it may be a potential therapy for addiction and craving[24-26].
In this study, under the condition of heroin cue exposure plus acupuncture at theZusanlipoint without twirling of the needle, the range of brain activation was not significantly different in either group compared with under heroin cue exposure, whereas the extent of brain activation did differ in the heroin addicts group. This suggests that acupuncture therapy at theZusanlipoint did not significantly affect brain activation in the control group, but oxygen consumption and brain blood flow in the heroin addicts group were higher. Under the state of heroin cue exposure and acupuncture at theZusanlipoint plus twirling of the needle, the range and degree of brain region activation were significantly different. Brain regions such as the cingulate gyrus, insular lobe, and thalamus did not show any activation. This is evidence that acupuncture at theZusanlipoint with twirling of the needle reduced the range of craving-related activation as well as the brain blood flow and neural activity in the activated regions. This evidence collectively suggests that acupuncture at theZusanlipoint plus twirling of the needle can rapidly suppress craving-related brain activation, and this may contribute to therapeutic mobilization of attentional, cognitive, and affective processing.
This study provides preliminary evidence that acupuncture at theZusanlipoint plus twirling of the needle can suppress activation in craving-related brain regions, and provides a reliable visualization reference for acupuncture as an intervention for drug addiction and craving. Acupuncture may cause hemodynamic and metabolic changes that interact between multiple functional areas of the brain, and influence the neural activity[27]. Because the fMRI signal reflects the oxygenation status of the blood, it has certain advantages in the localization of different brain regions. To understand the mechanisms underlying the effects of acupuncture on cue-induced craving, future research should apply cellular, electrophysiology, and molecular biology techniques.
SUBJECTS AND METHODS
Design
A synchronized, non-randomized, and controlled experiment.
Time and setting
Experiments were performed from September 2009 to December 2010 in Digital Imaging Center of the First Affiliated Hospital of Anhui College of Traditional Chinese Medicine, China.
Subjects
Functional data were collected in 24 participants. The heroin addicts group was composed of all men, aged 26–47 years. Their heroin consumption was 0.1–1.0 g per day, 0.78±0.35 g on average. Their duration of abuse was 3–214 months, and 97.7±69.2 months on average. The subjects in the control group were all men, and their average age was 35.4±3.6 years.
Diagnostic criteria for heroin addiction
The heroin addicts were diagnosed according to the diagnostic criteria for opioid dependence in theDiagnostic and Statistical Manual of Mental Disorders(DSM IV-R) published by the American Psychiatric Association and in theChinese Classification and Diagnostic Criteria of Mental Disorders-Revised(SSMD-III-R)[28-29]. The diagnostic criteria for opioid dependence included drug use lasting more than 1 month, unusual medication, and visible withdrawal symptoms.
Inclusive criteria for the heroin addicts group
(1) Subjects met the diagnostic criteria; (2) Subjects present with a protracted abstinence syndrome, such as varying degrees of sleep disorders, agitation, depression, or pain.
“我也这么认为过。”英格曼站下脚,回过头对闭着的大门说,“后来发现,对你们来说,激怒不激怒,结果都一样。”
Exclusive criteria for heroin addicts group
(1) Subjects with serious life-threatening diseases in the cardiovascular, cerebrovascular, liver, kidney and hematopoietic systems or mental diseases; (2) Subjects with a critical illness, or with visual or auditory disorder; (3) Subjects carrying magnetic ion substancesin vivo, such as a cardiac pacemaker, artificial valve, or metal foreign body residing in a vital organ; (4) Epilepsy patients; (5) Claustrophobic patients.
Inclusive criteria for the control group
(1) Subjects do not meet the above diagnostic criteria; (2) Subjects with no serious life-threatening diseases of the cardiovascular, cerebrovascular, liver, kidney, or hematopoietic system as well as no mental diseases; (3) Subjects with no visual, auditory, or language disorders; (4) Subjects without claustrophobic symptoms.
Exclusive criteria for control group
In addition to the exclusive criteria for heroin addicts group, opioid addicts were excluded.
This study used acupuncture therapy, which has been confirmed to be safe and effective in improving protracted withdrawal symptoms in clinical applications and in research. In accordance with the “Helsinki Declaration” and the Management Guidelines for Clinical Drug Research, informed consent was acquired. Prior to the recruitment of each participant, physicians gave a complete and comprehensive introduction of the study objectives, experimental methods, and possible adverse reactions in written form. In addition, patients were informed that they had the right to withdraw at any time.
According to theAdministrative Regulations of Medical Institutions, formulated by the State Council of the China[30], written informed consent was obtained from the participants for their involvement in this study and with their understanding of the detection scheme and risks.
Methods
Cue exposure and acupuncture intervention
In the preparation room, the participants changed clothes and were kept relaxed for 30 minutes before scanning. Then, they were required to close their eyes and lie in the scanner. Their ears were blocked with cotton balls and a special soundproof ear mantle. The participants’ heads were held rigid to reduce motion. The ambient lighting was turned off to reduce visual stimulation. All sounds except system noises from the equipment were eliminated to minimize disturbances. The participants were asked to keep their whole body, especially their head, stationary and avoid psychological activities as much as possible.
The task included several parts as follows: A restingstate for 1 minute, presentation of heroin cues for 1 minute (i.e., seeing heroin in a transparent glass bottle), presentation of the heroin cues plus acupuncture at the bilateralZusanlipoint for 1 minute, and presentation of the heroin cues plus acupuncture at theZusanlipoint and twirling of the needle for 1 minute. The heroin was provided by the Anti-Drug Abuse Office, Public Security Ministry of Anhui Province, China. All fMRI scanning was carried out after the task. The above procedures were repeated three times with an interval of 1 minute of rest between each repetition.
Acupuncture at theZusanlipoint (angle 90°, depth 2.5 cm) was performed by an experienced acupuncturist. Briefly, a disposable and sterile stainless steel filiform acupuncture needle (Suzhou Medical Equipment Co., Ltd., Suzhou, Jiangsu Province, China) was inserted into left and rightZusanlipoints. Two manipulations were used. The first manipulation is called non-twirled acupuncture, and involved inserting the needle into the points to an approximate depth of 2.5 cm without twirling of the acupuncture needle. The other is called twirled acupuncture, which was the same as non-twirled acupuncture, except it involved the reinforcing-reducing method, in which the needle was rotated in the left and right direction at a frequency of 60 times/min.
Brain fMRI scanning
Images were collected using a 1.5T Siemens Symphony scanner (Siemens, Germany) with a standard quadrature head coil (Siemens). A total of 6 sequences were acquired. The scanning timeline was as follows: (1) positioning imaging; (2) T2-weighted imaging to rule out other neurological symptoms; (3) T1 anatomical imaging in the axial plane parallel to the anterior commissure -posterior commissure line, over a total of 36 layers that covered the whole brain, with a field pattern collected to correct for magnetic field inhomogeneities; (4) functional imaging with an echo planar imaging blood oxygenation level dependent (BOLD) sequence in the same locations as the anatomical imaging repetition time (TR)=4 000 ms, echo time (TE)=50 ms, flip angle (FA)=90°, field of view (FOV)=192 mm×192 mm, matrix=64×64, resulting in 36-layer whole-brain images with 0.75 mm slice gap per 0.4 second); (5) inversion recovery T1 weighted 3D anatomical scanning (TR=2 100 mm, TE = 3.93 mm; FA=13°; FOV=250 mm×250 mm; slice thickness/spacing=1.0 mm/0.5 mm; matrix=256×256); (6) spin-echo T1-weighted anatomical imaging (TR=500 ms, TE=12 ms; FOV=230 mm×230 mm; slice thickness/spacing=3.0 mm/0.75 mm; matrix=192×144). This full time course of scanning took about 30 minutes.
Brodmann area
Based on the Brodmann area system, the cerebral cortex was divided into several brain regions, represented by numbers. The signal intensity under heroin cue exposure and under resting-state conditions was compared using theTstatistic.
X-axis: BOLD image number; Y-axis: head motion (mm) or angle (°); ΔA-P: anterior-posterior movement; ΔR-L: right-left movement; ΔI-S: inferior-superior movement; Yaw: rotation along Y-axis; Pitch: rotation along X-axis; Roll: rotation along Z-axis.
fMRI data analysis
The location of the activated brain regions, by Brodmann area partition, and T values were provided by the Digital Imaging Center of the First Affiliated Hospital of Anhui College of Traditional Chinese Medicine, China. The imaging data were analyzed using Analysis of Functional Neuroimages software (AFNI; http://afni.nimh.nih.gov/ afni/). The data processing consisted of 4 steps: (1) data format conversion; (2) calculation of individual statistical parametric mapping; (3) analysis of brain activation areas; (4) display threshold correction. The Monte Carlo simulation method was used and aP<0.05 was defined as the boundary value, to calculate the minimum data cluster size ofα<0.05. After the threshold was corrected, we analyzed and recorded the locations of the activated areas, using Brodmann partitioning, the signal type, and the location coordinates. These results are presented as brain activation profiles.
Funding:This study was supported by the Natural Science Foundation of Anhui University of Traditional Chinese Medicine, No. 2011zr001A and the Key Project for Science and Technology of Anhui Province, No. 07010302205.
Author contributions:Xinghui Cai was responsible for the research concept and design, drafted the manuscript, and acquired funding from the Natural Science Foundation of Anhui University of Traditional Chinese Medicine. Xiaoge Song provided training, validated the paper, and acquired funding from the Key Project for Science and Technology of Anhui Province. Chunsheng Xu, Chuanfu Li and Qi Lu provided technical support, performed data analyses, and were in charge of statistical processing. Xiliang Li integrated the data and wrote the manuscript.
Conflicts of interest:None declared.
Ethical approval:This experiment was approved by the Ethics Committee, the First Affiliated Hospital of Anhui University of Traditional Chinese Medicine in China.
Author statements:The manuscript is original, has not been submitted to or is not under consideration by another publication, has not been previously published in any language or any form, including electronic, and contains no disclosure of confidential information or authorship/ patent application disputations.
[1] Garavan H, Pankiewicz J, Bloom A, et al. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157(11): 1789-1798.
[2] Xie CM. Visual evoked heroin addict brain electrical activity changes. Zhonghua Shenjingke Zazhi. 2006;39(1): 61.
[3] Liu GX, Yang Z, Liu XF, et al. Heroin addicts’ attentional bias to heroin-related cues. Zhongguo Xinliweisheng Zazhi. 2009;23(9):677-679.
[4] Fan CL, Zhao M, Du J, et al. Physiological reactions to heroin-related cues in abstinent heroin dependent patient. Zhongguo Yaowu Yilaixing Zazhi. 2009;18(2): 118-122.
[5] Zhang XL, Zhao LY, Sun LL, et al. Effects of drug related cue on decision-making in heroin addicts at different abstinent time. Zhongguo Yaowu Yilaixing Zazhi. 2009; 18(4):368-370.
[6] Bossert JM, Ghitza UE, Lu L, et al. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526(1):36-50.
[7] Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis:neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642-1652.
[8] Royall DR, Lauterbach EC, Cummings JL, et al. Executive control function:a review of its promise and challengesfor clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci. 2002;14(4):377-405.
[9] Kaufman JN, Ross TJ, Stein EA, et al. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci. 2003;23(21):7839-7843.
[10] Zheng JL, Wu YM, Shu SY, et al. Role of the parietal lobes in cognition of the spatial memory in healthy volunteers. Tianjin Yiyao. 2008;36(2):81-83.
[11] Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167-202.
[12] Wexler BE, Gottschalk CH, Fulbright RK, et al. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158(1):86-95.
[13] Wehner F, Wehner HD, Subke J, et al. Demonstration of morphine in ganglion cells of the hippocam pus from victims of heroin over-dose by means of anti-morphine antiserum. Int J Legal Med. 2000;113(2):117-120.
[14] Daniel WH. Functional imaging of craving. Alcohol Res Health. 1999;23(3):187-197.
[15] Schutter DJ, van Honk J. The cerebellum on the rise in human emotion. Cerebellum. 2005;4(4):290-294.
[16] Long Y, Liu B, Liu X, et al. Resting-state functional MRI evaluation of after-effect of acupuncture at Zusanli point. Zhongguo Yixue Yingxiang Jishu. 2009;25(3):373-376.
[17] Liu B, Liu X, Chen J, et al. Study on the effects of acupuncture at acupoint and non-acupoint on functional connectivity of different brain regions with functional magnetic resonance imaging. Zhongguo Zhen Jiu. 2009; 29(12):981-985.
[18] Song XG, Li CF, Huang JJ, et al. Acupuncture for clues induced heroin abuser that the influence of functional magnetic resonance imaging (fmri) preliminary observation. Anhui Zhongyi Xueyuan Xuebao. 2008;27(4): 21-22.
[19] Fu P, Jia JP, Xu M, et al. Changes of brain function in different areas of cerebral cortices due to electroacupuncture at the point ST36 through MRI. Zhongguo Linchuang Kangfu. 2005;9(16):92-94.
[20] Yin L, Jin XL, Shi X, et al. Acupuncture at the point ST36 PET and fMRI brain imaging of the initial study. Zhongguo Kangfu Lilun yu Shijian. 2002;8(9):523-524.
[21] Wu JM, Wei DY, Luo YF, et al. Clinic reaseach on heroin de-addiction effects of acupuncture and its potentiality of preventing relapse. Zhongxiyi Jiehe Xuebao. 2003;1(4): 268-272.
[22] Xu P, Jiang YP, Wang Y, et al. Influence of acupuncture on heroin addicts attentional bias. Shanghai Zhongyiyao Zazhi. 2006;40(3):40-41.
[23] Gao YJ, Yang H, Yang LM, et al. Acupuncture combined with psychological rehabilitation ease heroin addicted patients who craves psychological clinical observation. Shizhen Guoyi Guoyao. 2007;18(6):1489-1490.
[24] Shi GX, Wang DJ, Liu CZ, et al. The analysis of the factors that influence acupuncture effect. Zhongyi Zazhi. 2009;50(10):942-944.
[25] Zhang YN, Yang S, Pan XN, et al. Experimental study on the influence of acupoint and duration of acupuncture on the effect. Tianjin Zhongyiyao. 2010;27(2):118-120.
[26] Zhang TS, Guan F, Jin CN, et al. Overview of relation between retaining time of acupuncture and effect for apoplexy. Zhen Ci Yan Jiu. 2009;34(2):140-142.
[27] Zhang R, Zou YQ, Huang SQ, et al. MRI cerebral function imaging following acupunctureat Hegu, Zusanli, Neiguan and Sanyinjiao points. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2007;11(22):4271-4274.
[28] Liu TT, Shi J, Epstein DH, et al. A meta-analysis of chinese herbal medicine in treatment of managed withdrawal from heroin. Cell Mol Neurobiol. 2011;20(4): 301-307.
[29] Liu TT, Shi J, Bao YP, et al. Systematic review of effectiveness and safety of chinese medicine combined with western medicine in treatment of opioid withdrawal syndrome. Zhongguo Yaowu Yilaixing Zazhi. 2008;14(6): 336-337.
[30] State Council of the People’s Republic of China. Administrative Regulations on Medical Institution. 1994-09-01.
10.3969/j.issn.1673-5374.2012.33.006 [http://www.crter.org/nrr-2012-qkquanwen.html]
Cai XH, Song XG , Li CF, Xu CS, Li XL, Lu Q. Acupuncture inhibits cue-induced heroin craving and brain activation. Neural Regen Res. 2012;7(33):2607-2616.
Xinghui Cai★, Master, Lecturer, School of Acupuncture and Orthopedics, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
Xiaoge Song, Researcher, Institute of Acupuncture and Meridians, Anhui University of Traditional Chinese Medicine, Hefei 230038, Anhui Province, China
2012-08-08
2012-10-17 (N20120622001/WJ)
(Edited by Chen YG, Wu XG/Yang Y/Wang L)
- 中国神经再生研究(英文版)的其它文章
- Electroacupuncture improves neuropathic pain Adenosine, adenosine 5’-triphosphate disodium and their receptors perhaps change simultaneously☆
- Underlying mechanism of protection from hypoxic injury seen with n-butanol extract of Potentilla anserine L. in hippocampal neurons***☆
- Shuanghuanglian injection downregulates nuclear factor-kappa B expression in mice with viral encephalitis*★
- Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice**★
- Puerarin prevents high glucose-induced apoptosis of Schwann cells by inhibiting oxidative stress*★
- Heat-sensitive moxibustion attenuates the inflammation after focal cerebral ischemia/ reperfusion injury*☆

