圣和散对神经胶质瘤细胞DNA放射损伤修复的抑制作用
王 欢1,王建华2,侯增霞3
(1.山西中医学院,山西太原030024; 2.第四军医大学西京医院,陕西西安710032;3.第二军医大学长海医院,上海200433)
High-grade glioma is a common primary malignant brain tumor.Standard therapy consists of surgical resection followed by radiotherapy.The prognosis in patients is very poor with conventional therapeutic modalities[1-2].Attempts to improve outcome by increasing the local radiation dose have in general been unsuccessful because of necrosis in normal cells adjacent to tumor,which becomes dose limiting before significantly improved local tumor control is achieved.On conventional radiation dose,the development of resistance of glioma to radiation is a major problem during the treatment of tumors[3-4].Obviously,a more effective approach would be to develop agents that sensitize these glioma cells to radiation selectively.Studies approved DNA repairing capacity related to radiosensitivity and cytotoxicity induced by radiation[5].To demonstrate therapeutic mechanism of SHP on glioma,DNA damage and repair in C6 and NHA treated with radiation were examined.
1 Materials and Methods
1.1 Materials
Glioma cells of rats were purchased from the Fourth Military Medical University,normal human astrocytes were adopted from surgery.3-[4,5-dimethylthiozol-2-yl]-2,5-diphenyltetrazolium bromide(MTT,Sigma Chemicals,St Louis,MO),anti-γH2AX antibody(Upstate Biotechnology,USA).SHP mainly includes Radix Ginseng,Scrophularia ningpoensis Hemsl,A -tractylodes macrocephala Koidz,Lacrymajobi Linn.var.ma-yuen(Roman.)Stapf,Herba Hedyotis Diffusae,Herba Cistanches,Bufo gargarizans,and Glycyrhiza uralensis Fisch.All herbs were purchased from Shaanxi Company of Chinese Herbal Medicines and identified by professor Xia Yuesheng.
1.2 Methods
1.2.1 Extraction of SHP
The dried whole herbs(1 000 g)were degreased by heating under reflux with industrial ethanol in a bath and then extracted 3 times with 10 L boiling distilled water for 1 h each time.The decoction was then filtered through carbasus,mixed,and concentrated to 1 000 mL.The concentrated extract was mixed with 95%ethanol to make the content of ethanol up to 80%.After standing overnight(20±2 h),the precipitate was filtered,washed,and vacuum-dried to form a brown powder.Before the experiment,it was dissolved in phosphate-buffered saline(PBS)and filtered with a 0.22 μm membrane.The filter liquor was made up to concentrations of 1.0 mg/L and stored at 20℃.
1.2.2 Cell Culture
C6 and NHA cells were grown in RPMI 1640(Life Technologies,Inc.,Rockville,MD)containing glutamate(5 mM)and 5%fetal bovine serum,and maintained at 37℃in an atmosphere of 5%CO2and 95%room air.
The cell lines grown exponentially of C6 and NHA were treated with SHP(1 mg/L)for 2 h.Then,culture plates were cooled on ice and irradiated with 0 Gy~2 Gy of gamma radiation at a dose rate of 0.78 Gy/min(137cesium irradiator,Atomic Energy,Ottawa,Canada).SHP was not removed from the medium.After irradiation,NHA and C6 cell lines were replaced into the incubator(37°C)and the kinetics of DNA repair was assessed,control cells were subjected to similar treatment but without irradiation or SHP.
1.2.3 Inhibitive effect of SHP on cells growth
C6 and NHA Cells were plated in a 96-well plate and treated with lower concentrations of SHP(1 mg/L),and some were exposed to radiation.After treatment,cells were stained with MTT,and incubated for 4 h in 37℃incubator.Then the cells were lysed in 150 μL mixture of ethanol/DMSO(1∶1),and the absorbance was detected by a 96-well plate reader at 540 nm.
1.2.4 Damage and repair of DNA
C6 and NHA cells were cultured on coverslips placed in 35 mm dishes.Fractional cells were exposed to 1 mg/L SHP for 24 h at 37 ℃,then the cells were rapidly washed 3 times with PBS and incubated in fresh medium for 30 min to give sufficient time for phosphorylation of histone H2AX.Other cells were exposed to 1 mg/L SHP for 2 h at 37 °C and then irradiated with 0 Gy,1 Gy,or 2 Gy and incubated for a further 30 min.The medium was removed,and the cells were washed with PBS and then held in methanol/PBS(50∶50 v/v)at room temperature for 10 min before fixing in methanol at 30℃for 30 min.After removal of the methanol,the cells were held in PBS at room temperature for 5 min and then blocked with 5%milk powder in PBS for 30 min.The cells were then stained with antiphospho-histone H2AX antibodies for 1.5 h at room temperature in the dark.After removal of the primary antibody,the cells were washed with PBS,followed by 0.1%Tween 20 in PBS and 3 more times with PBS,and then incubated with Alexa Fluor 488-labeled goat anti-mouse IgG antibody(Molecular Probes,Eugene,OR)at room temperature in the dark for 45 min.The cells were again washed with PBS,followed by 0.1%Tween 20 in PBS and 3 more times with PBS and rinsed with water.The coverslips were mounted on microscope slides with 4 μL mounting solution(1 mg/mL p-phenylenediamine,3 μg/mL 4′,6-diamidino-2-phenylindole[DAPI],90%glycerol in PBS)and stored at 4℃in the dark.Fluorescent foci were imaged with a Zeiss LSM510 Laser Scanning Confocal microscope(Carl Zeiss,Jena,Germany)mounted on a Zeiss Axiovert 100M microscope.Images were captured by a Photometrics Sensys CCD camera(Roper Scientific)and imported into IP Labs image analysis software package(Scanalytics,Inc.).Foreach treatmentcondition,γH2AX foci were determined in at least 300 cells.
1.2.5 Statistical Analysis
Data were presented as(mean±SD,or SE).Mean values were compared by the Student′s t test.The threshold of statistical significance was set at P<0.05.Statistical analyses were performed using SPSS17.0 package(SPSS,Chicago,IL,USA).
2 Results
2.1 Proliferation Assay
C6 and NHA cells were treated with SHP at 1 mg/L,24 h before single-dose irradiation(0 Gy,1 Gy or 2Gy).The MTT assay was used to determine the effect of SHP on C6 and NHA cells survival and proliferation at 0 d,2 d,4 d,and 6 d after the cells were irradiated.As figure 1 showed,both cell lines when irradiated,but not treated with SHP,showed a time-dependent decrease in cell survival and proliferation compared with cells that received no radiation(P<0.005),the C6 cell line seems more radioresistant than the NHA cell line.NHA cell lines treated with SHP and radiation showed a time-dependent decrease in cell proliferation before 2 d,which gradually presented radioresistant compared with cells that received radiation alone after 2 d (P<0.005)(Figure 1A).Figure 1 displayed the largest decline in cell survival occurred between 2 d and 4 d after radiation.Both cell lines treated with SHP did result in an opposite results.C6 proliferation and cell viability decreased significantly after SHP,the greatest decrease in cell survival and proliferation occurred in the 4th d(P<0.001).Contrary,NHA treated with SHP reversed the shape of the timedependent decrease curve and upgrade the curve.It implied that SHP may inhibit the repair of DNA damage in C6 and enhance the repair of DNA damage in NHA.
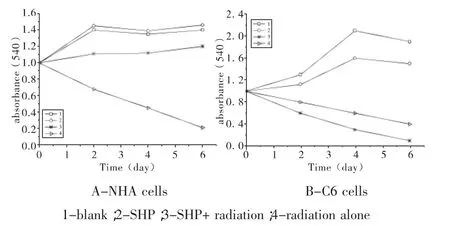
Figure 1 Proliferation and survival in rat glioma cell lines(C6)and normal human astrocytes(NHA).The MTT assay was done 0 d,2 d,4 d,6 d after treatment.
2.2 H2AX Phosphorylation in Glioma and Normal Astrocytes Cells
Immunofluorescence was used to visualize and quantify phosphorylated H2AX foci in C6 and NHA cells at various time after irradiation in vitro.These foci were thought to represent sites of DNA doublestrand breaks(DSBs)where chromatin structural change is occurring.H2AX phosphorylation is recognized to be an early event in break repair and has a role in subsequent recruitment of repair proteins.Foci resolution is thought to occur following DSBs repair,and persistent foci at late time points are thought to represent residual unrepaired DSBs[6-7].By comparing the number of foci per cell across the dose range of interest over a repair time from 30 min to 24 h,we can compare induction and resolution of DSBs and therefore define DSBs repair kinetics in these cell lines.
We used the assay in the C6 and NHA cells examined at 0.5 h,4 h,8 h,and 24 h after irradiation,when resolution of foci is occurring.These data were expressed as foci resolution with time;a representation of DSBs repair kinetics is shown in Figure 2.Clearly much higher amounts of γH2AX foci were observed in NHA control group and C6 treated group.All cells tested showed radiation dose-dependent increase in residual γH2AX foci reflecting unrepaired DNA dou-ble-strand breaks.However,SHP pretreatment further increased the number of residual γH2AX foci in C6,and continued to increase steadily up to 24 h.Higher amounts of γH2AX foci were observed in C6 compared with NHA cells(P<0.005).The remaining foci at 24 h are thought to represent unrepairable damage,which occurred at a rate of approximately two lesions per cell per gray.The data indicate that in C6 cells,more DSBs were produced in C6 and left unrepaired,whereas in NHA cells,less DSBs were produced and/or were subject to repair.The data are in line with the enhanced sensitivity of C6 cells compared with NHA cells.

Figure 2 Phosphorylated H2AX foci in the rat glioma cell lines(C6)and in normal human astrocytes(NHA)
3 Discussion
High-grade glioma remains virtually incurable with current therapeutic regimens,despite considerable advances during the last two decades in neurosurgical techniques,radiation,and chemotherapy,treatment of malignant gliomas remains mostly palliative,median survival is about 1 year from the time of diagnosis[2,8].To enhance the effects of radiation,two major strategies have been proposed.The first approach is based on the reduction of the treatment volume,the second strategy makes use of the increase of the differential response between the tumor and normal tissue to,for example,chemotherapeutic drugs,biologic agents,and genetic or proteomic techniques[9-11].However,complex molecular determinants of tumor response to radiation have limited the success of these approaches to date.Obviously,it is important to find a more effective approach which can develop agents that selectively sensitize these glioma cells to radiation.
We previously demonstrated that Shenghe powder could induce tumor cells apoptosis and reverse multidrug resistance[12],inhibit the proliferation of C6 and decrease malignant degree[13],improve the survival time of the patients with postoperative high-grade glioma[14].Its complex components may offer a novel antitumor mechanism,the objective of this study was to determine the effect of Shenghe powder on the repair of DNA damage induced by radiation.Radiation causes a broad spectrum of DNA lesions such as alkali-labile lesions,single-strand breaks and DSBs,DSBs is the major threat to the genomic integrity of cells,which is biologically the most significant lesions produced by radiation and other exogenous cytotoxic agents[15-17].It has been shown that histone H2AX becomes phosphorylated immediately after irradiation,which in turn is believed to recruit DNA repair factors to sites of DNA double-strand breaks[18-19].Because γH2AX is closely associated with DNA double-strand break repair mechanisms,we studied the radiation response of C6 and NHA lines in vitro,using γH2AX expression as a marker of DNA double-strand breaks.Of the various techniques currently available to assay double-strand breaks,we chose an immunofluorescence approach involving quantification of phosphorylation of histone H2AX because of its sensitivity,which can be readily detected by fluorescent immunostaining with antibodies to the phosphorylated form of the protein.The fluorescent intensity correlates well with the number of DNA double-strand breaks[20].This research showed that Shenghe powder enhanced radiosensitivity of C6 cells,the pre-treatment with Shenghe powder resulted in reduced numbers of γH2AX foci in irradiated C6 cells,the capacity of repair was markedly decreased.Contrarily,Shenghe powder could depress the radiation-induced DNA double-strand break and enhance the DNA repair capacity in NHA.
As the biological underpinnings of such phenomena as radioresistance are often multiple rather than uniface.The multiple factors reasons focus on seeking multi-approach to enhance radiosensitivity of glioma,include of down-regulation resistance genes or up-regulation of target genes.Shenghe powder,its complex components may offer multitarget treatment,which anastomose to requirement of multi-approach treatment in glioma.
In conclusion,our studies revealed that Shenghe powder has a novel bidirectional function:Shenghe powder diminished the formation of γH2AX foci in C6 cells exposed to radiation,decreased the capacity of DNA repair,enhanced radiosensitivity of C6 cells.Shenghe powder improved the radioresistance of normal human astrocytes and prevented normal astrocytes from radiation.Further experiments are necessary to confirm more mechanism.
[1]Fogh S E,Andrews D W,Glass J,et al.Hypofractionated stereotactic radiation therapy:an effective therapy for recurrent high-grade gliomas[J].J Clin Oncol,2010,28(18):3 048-3 053.
[2]Arslan M,Karadeniz A N,Aksu G,et al.Postoperative hypofractionated radiotherapy in glioblastoma multiforme[J].J Buon,2006,11(1):39-42.
[3]DeAngelis L M,Panageas K,Nolan C P,et al.Randomized phase II trial of chemoradiotherapy followed by either dose-dense or metronomictemozolomidefornewlydiagnosedglioblastoma[J].JClinOncol,2009,27(23):3 861-3 867.
[4]Stupp R,Chakravarti A,Hegi M E,et al.Chemoradiotherapy in malignant glioma:standard of care and future directions[J].J Clin Oncol,2007,25(26):4 127-4 136.
[5]Manti L,D′Arco A.Cooperative biological effects between ionizing radiation and other physical and chemical agents[J].Mutat Res,2010,704(1-3):115-122.
[6]Mah L J,El-Osta A,Karagiannis T C. γH2AX:a sensitive molecular markerofDNAdamageandrepair[J].Leukemia,2010,24(5):679-686.
[7]Entin-Meer M,Yang X,VandenBerg S R,et al.In vivo efficacy of a novel histone deacetylase inhibitor in combination with radiation for the treatment of gliomas[J].Neuro Oncol,2007,9(2):82-88.
[8]Sathornsumetee S,Rich J N.New treatment strategies for malignant gliomas[J].Expert Rev Anticancer Ther,2006,6(7):1 087-1 104.
[9]Muni R,Minniti G,Lanzetta G,et al.Short-term radiotherapy followed by adjuvant chemotherapy in poor-prognosis patients with glioblastoma[J].Tumori,2010,96(1):60-64.
[10]Bidros,D S,Liu J K,Vogelbaum M A.Future of convection-enhanced delivery in the treatment of brain tumors[J].Future Oncol,2010,6(1):117-125.
[11]Both G W.Recent progress in gene-directed enzyme prodrug therapy:an emerging cancer treatment[J].Curr Opin Mol Ther,2009,11(4):421-432.
[12]Wang J,Xia Y,Wang H,et al.Chinese herbs of Shenghe Powder reverse multidrug resistance of gastric carcinoma SGC-7901[J].Integr Cancer Ther,2007,6(4):400-404.
[13]Xia Y,Wang J,Wu Y,et al.Inducing differentiation effect of Shenghe Powder on C6 brain glioma cells[J].Lishizhen Med Materia.Medica Res,2007,18(2):290-291.
[14]Xia Y.Role of Shenghe Powder in Radiation on malignant glioma after surgery[J].J G.M.Tradit.Chinese Med,2005,20(5):50-51.
[15]Lavin M F,Birrell G,Chen P.ATM signaling and genomic stability in response to DNA damage[J].Mutat Res,2005,569:123-132.
[16]Singh S K,Wu W,Wang M,et al.Extensive repair of DNA double-strand breaks in cells deficient in the DNA-PK-dependent pathway of NHEJ after exclusion of heat-labile sites[J].Radiat Res,2009,172(2):152-164.
[17]Sarcar B,Kahali S,Chinnaiyan P.Vorinostat enhances the cytotoxic effects of the topoisomerase I inhibitor SN38 in glioblastoma cell lines[J].J Neuro Oncol,2010,99(4):201-207.
[18]Kanaar R,Hoeijmakers JHJ,van Gent D C.Molecular mechanisms of DNA double-strand break repair[J].Trends Cell Biol,1998,8(12):483-489.
[19]Momota H,Ichimiya S,Kondo N.Histone H2AX sensitizes glioma cells to genotoxic stimuli by recruiting DNA double-strand break repair proteins[J].Int J Oncol,2003,23(2):311-315.
[20]Sedelnikova O A,Rogakou E P,Panyutin I G,et al.Quantitative detection of(125)IdU-induced DNA double-strand breaks with gamma-H2AX antibody[J].Radiat Res,2002,158(4):486-492.
——第二届中国空间科学大会在山西太原举行
———记第二军医大学免疫学研究所副教授侯晋

