Determination of 3-Monochloropropane-1,2-diol in Soy Sauce and Oyster Sauce by Solid Phase Extraction Combined with Gas Chromatography-Mass Spectrometry
XIONG Jun,GONG Liang,LAI Yi-dong
(Guangdong Dongguan Quality Supervision and Testing Center, Dongguan 523808, China)
Determination of 3-Monochloropropane-1,2-diol in Soy Sauce and Oyster Sauce by Solid Phase Extraction Combined with Gas Chromatography-Mass Spectrometry
XIONG Jun,GONG Liang,LAI Yi-dong
(Guangdong Dongguan Quality Supervision and Testing Center, Dongguan 523808, China)
Abstract :A simple and sensitive method for determination of 3-monochloropropane-1,2-diol (3-MCPD) in sauce samples by solid phase extraction (SPE) coupled with with gas chromatography-mass spectrometry (GC-MS) is described. In this work, elution solvent type and amount and sample loading amount were investigated to optimize SPE conditions. The optimal sample preparation procedure for treating 5.0 g of samples involved homogenization with 5 mol/L sodium chloride solution, clean-up on SPE column and derivitization prior to GC-MS analysis. The limit of detection of the method for 3-MCPD was 0.15μg/kg, and the linear range 0.51-6144μg/kg, with a correlation coefficient of 0.9998 and a relative standard deviation of 8.8% (RSD, n = 5). The method was applied to determine soy sauce and oyster sauce samples and spiked recoveries of 87.2%-109.4% with RSDs (n = 3) of 5.6%-10.2% were obtained.
Key words:solid phase extraction;3-monochloropropane-1,2-diol;gas chromatography-mass spectrometry (GC-MS);sauce samples
3-Monochloropropane-1,2-diol (3-MCPD) was first detected in acid-hydrolyzed vegetable protein (HVP) by the reaction of hydrochloric acid with residual vegetable lipid and had been obtained more and more attention during the last few decades due to its carcinogenic effects[1]. Several studies have showed that 3-MCPD exists in a wide variety of food during food processing such as nontraditionally prepared soy sauce, vinegar and so on, its formation is origin from glycerol or acylglycerols and chloride ions and influenced by a series of factors including moisture, lipid content, pH value and food type[2-4]. Therefore, it is necessary to set safe levels of consumption to protect human health from the adverse effects of 3-MCPD. Commission Regulation (EC) No 1881/2006 of 19 December 2006[5]sets the tolerable daily intake (TDI) at 2μg/kg bw. Besides, the maximum permitted concentration of 3-MCPD in foodstuffs are controlled by legislation. For example, maximum levels in foodstuffs (in particular HVP and soy sauce) are 20μg/kg for a liquidproduct containing 40% dry matter, while a maximum level of 50μg/kg in dry matter. In view of the situation a sensitive, fast, simple and accurate method of analysis is required and it is necessary to determine its level in food and to ensure it is within the permitted limits.
However, it is difficult to analyse 3-MCPD sensitively due to its high boiling point and absence of a suitable chromophore. At present, the determination of 3-MCPD in food samples is mainly carried out by gas chromatography (GC) with a variety of detectors including flame ionization detector (FID)[6], electron capture detector (ECD)[7-8]and mass spectrometry (MS)[7,9-13]. Among the above detectors, FID and ECD are subject to interference, which makes the qualitative analysis difficult. Relatively speaking, GC-MS is the most powerful technique for the analysis of 3-MCPD due to its high selectivity and high accuracy. Because of high polarity and low volatility, 3-MCPD needs derivatisation. Heptafluorobutyrylimidazole (HFBI), heptafluorobutyric acid anhydride (HFBA), boronic acid, ketones, N,O-bis (trimethylsilyl) trifluoroacetamide (BSTFA) derivatives are usually used as derivatization reagents.
To date, in order to detect low levels of the analytes, a preconcentration step is needed prior to instrumental determination. Liquid-liquid extraction (LLE)[8]and solid-phase extraction (SPE)[6-7,9-12]were the most popular sample pretreatment techniques. But LLE has many drawbacks such as time consumption, being labour-intensive and the use of large volumes of solvent, which often leads to the formation of emulsions. In addition, a large volume of sample is often required due to the low concentration of analytes in the samples. SPE is less time-consuming compared with LLE. In recent years, methods based on SPE, derivatisation and subsequent GC-MS analysis usually enable quantification of 3-MCPD at theμg/kg level[6-7,9-12]. For example, SPE-GC-MS for the determination of 3-MCPD in foods and food ingredients using HFBI as a derivatization reagent was purposed by Brereton et al[6].
In the present work, the effective and sensitive method based on SPE has been developed for the determination of 3-MCPD in sauce samples by GC-MS. To obtain an optical condition for the extraction of 3-MCPD, a serious of influencing factors including type of elution solvent, volume of elution solvent and sample have been investigated. The developed method has been validated by the analysis of 3-MCPD in real samples.
1 Materials and Methods
1.1Reagents and materials
3-MCPD and HFBI were obtained from Thermo Fisher Scientific (Fair Lawn, NJ, USA). Their purity was above 98.0%. Hexane, ethyl acetate and ether were purchased from Sinopharm Chemical Reagent Co. Ltd., (Shanghai, China). All reagents used were at least of analytical reagent grade. Doubly distilled water was used throughout this work. Standard stock solution (1.280 mg/mL) of 3-MCPD was prepared in hexane and stored in refrigerator. Working solutions used in further studies were prepared freshly by diluting different amounts from the standard solution with doubly distilled water to the required concentrations. All solutions were stored at 4 ℃ in a refrigerator prior to use.
1.2Preparation of samples
The soy sauce and oyster sauce samples were purchased from a local supermarket in Dongguan, China. About 5.000 g of soy sauce sample was weighed into a glass tube, and 5 mol/L NaCl solution was added until the mixture solution was 10 mL. Then, the tube was tightly closed and mixed on the vortex meter for 3 min. Subsequently, the mixture solution was directly processed according to SPE procedure under the optimized condition.
For the oyster sauce sample, the preparation process of weight and mixture was the same to the soy sauce. Then, the mixture solution was centrifuged for 5 min at 6000 r/min due to its complicated matrix. Finally the upper solution was directly processed according to SPE procedure under the optimized condition.
For the recovery study, the samples were prepared by spiking a known amount of the target analyte, and processed according to SPE procedure under the optimized condition.
1.3Procedure
The SPE column using 15 mL (3000 mg) of the MN Chromabond-XTR was not conditioned. 3.0 mL of the sample mixture solution passed through the column. After 30 min, the column was leached with 10.0 mL of n-hexane and then eluted by 12.0 mL of the mixture solution of ethyl acetate and ether (9∶1,V/V) at a flow rate of 1.0 mL/min. The elution solution was collected and concentrated under a gentle nitrogen flow. The residue was redissolved in 1.0 mL n-hexane and derivated with HBFI at 70 ℃ for 20 min in the subsequent. After cooling to room temperature, 2 mL of 5 mol/L NaCl solution was added to remove the excess derivatization agent. The nhexane layer was separated and analyzed by GC-MS.
1.4GC-MS analysis
Chromatographic analysis was made on an Agilent 6890N gas chromatograph equipped with 5975B mass spec-trometry (MS) system (Agilent Technologies, Palo Alto, CA, USA). A DB-5MS capillary column (30 m × 0.25 mm id and 0.25μm film thickness) purchased from J & W Scientific (Folsom, CA, USA) was employed. The injection was made in the splitless mode at 250 ℃. Helium was used as carrier gas with constant flow of 1.0 mL/min. The column oven temperature was as follows∶ first held at 50 ℃ for 1 min, then programmed at 5 ℃/min to 90 ℃, finally 50 ℃/min to 250 ℃ and held for 6.0 min. The GC-MS was operated in the electronimpact mode at 70 eV with the transfer line temperature of 280 ℃ and an ion source trap temperature of 200 ℃. Full scan data acquisitions were carried out over the mass range m/z 40 -500. Qualitative and quantitative analysis was carried out by selectively monitoring the detector response of characteristic molecular ions at m/z 253, 275, 289, 291, 453 for the derivative of 3-MCPD.
2 Results and Analyses
In order to obtain best sensitivity, different parameters affecting on SPE including elution solvent type, the volume of elution solvent and sample have been optimized and established.
2.1Optimization of solid phase extraction
2.1.1Effect of elution solvent type

Fig.1 Effect of elution solvent on SPE
Careful attention should be paid to the selection of the elution solvent, which is very important for achieving good selectivity of the target compound. The elution solvent should fulfill the following requirements∶ First, it should have high elution efficiency; Second, it should not interfere with analyte and have low toxicity; Third, it should have compatibility with GC system. Hence, ether, ethyl acetate, and the mixture solution of ethyl acetate and ether have been investigated. Fig.1 was the effect of elution solvent on the recovery of target. As could be seen, compared with other elution solvents ether showed relative poorer elution efficiency for 3-MCPD, while, the mixture solvent of ethyl acetate and ether (9∶1, V/V) gave the best elution efficiency for the tested target analyte. Therefore, the mixture solvent of ethyl acetate and ether (9∶1, V/V) was selected as the elution solvent in subsequent experiments.
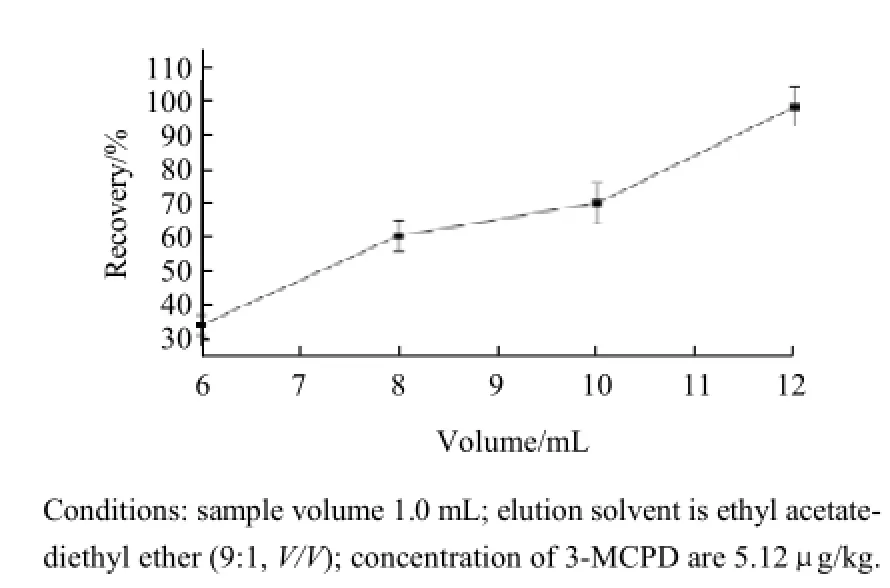
Fig.2 Effect of the volume of elution volume
2.1.2Effect of volume of elution solvent
SPE will attain the maximum sensitivity after the target analyte has been completely eluted. Therefore, the effect of the volume of elution solvent on the recovery was investigated with the volume in the range of 6-12 mL. The result showed that the recovery of 3-MCPD increased with the increase the volume and 3-MCPD could be quantitatively recovered when the volume was 12 mL (Fig.2). To ensure acquisition of satisfied recovery and trade off the analytical speed and the sensitivity, a volume of 12 mL was employed as the volume of eluent solvent for the following experiments. 2.1.3Effect of breakthrough volume of SPE column

Fig.3 Effect of the volume of sample volume
Under the following constant conditions (elution solvent, the mixture solution of ethyl acetate and ether (9∶1,V/V); eluent solvent volume, 12 mL; flow rate, 1.0 mL/min), different volumes (1.0, 2.0, 3.0, 3.5 mL) of the standard solution of the studied analyte passed through the SPE. Fig.3 was the effect of the breakthrough volume of the sample solution on the 3-MCPD recovery. As could be seen, the recovery was not lower than 90% when the volume of sample was less than 3.5 mL. To obtain better recovery and sensitivity, a volume of 3.0 mL was used for further experiments.

Table 1 Analytical performance of SPE-GC-MS for the determination 3-MCPD
2.2Analytical performance of the SPE procedure
The linearity of calibration curve of SPE for the target compound was observed in the range of 0.51-6144μg/kg with the correlation coefficient of 0.9998, which showed good linearity. Under the optimal experimental conditions, the repeatability, expressed as relative standard deviation (RSD) for five replicate analyse, spiked at 0.76μg/L of the target compound, was 8.8%. The limit of detection (LOD, RSN=3) was 0.15μg/kg (Table 1). From the above data, the LOD data showed that the sensitivity of method was good enough to ensure reliable measurements.
Table 2 is the comparison of the limit of detection obtained by LLE, SPE and SPME for extraction and determination of 3-MCPD in real samples. As could be seen, the LOD obtained by this method is lower than that reported in references[7-14], comparable with that obtained in the reference[15], and the proposed SPE-GC-MS method is sensitive and effective.
2.3Application of the SPE to the real samples
Quantitative analysis of real sample was carried out by SPE mode. Fig.4 depicted typical chromatogram of the real sample obtained after SPE. Peak identification of the 3-MCPD in samples was based on the comparison with the retention time of standard compounds, characteristic molecular ions and was confirmed by spiking known standard compounds to the sample. Accuracy was calculated as the percentage recovery of known amounts of target analyte added to soy sauce and oyster sauce and subjected to the SPE method under the optimized conditions. The recovery was defined as the ratio of the concentration of analyte found to the concentration of analyte spiked. With the application of external standard method, the average concentration of target compound in sauce and oyster sauce were determined by the proposed method and the results were given in Table 3. It could be seen that the recoveries for the spiked real samples varied from 87.2% to 109.4% and the RSDs calculated from these experiments were from 5.6% to 10.2%. The results showed that recoveries were good for the analyte, thus illustrating the practical effectiveness of the method.

Table 2 Comparison of detection limits found in the literature for the determination of 3-MCPD in real samples

Table 3 Analytical results and recoveries of target analytes in samples by SPE-GC-MS

Fig.4 Chromatogram of real sample obtained by SPE-GC-MS under optimized conditions
3 Conclusion
The determination of 3-MCPD in soy sauce and oyster sauce samples by means of solid phase extraction (GC-MS) with gas chromatography-mass spectrometry is described. The proposed method has many practical advantages such as not condition, simplicity of the extraction, high sensitivity and an outstanding capacity of avoiding the necessity of separate sample cleanup and was applied to soy sauce and oyster sauce samples. The recovery ranging from 87.2%-109.4% with RSDs of 5.6%-10.2% were obtained for SPE. References:
[1]VELISEK J, DAVIDEK J, HAJSLOVA J, et al. Chlorohydrins in protein hydrolysates[J]. Z Lebensm Unters Forsch, 1978, 167(4)∶ 241-244.
[2]CREWS C, HOUGH P, BRERETON P, et al. Survey of 3-monochloropropane-1,2-diol (3-MCPD) in selected food groups, 1999 -2000[J]. Food Addit Contam, 2002, 19(1)∶ 22-27.
[3]COLLIER P D, CROMIE D D O, DAVIES A P. Mechanism of formation of chloropropanols present in protein hydrolysates[J]. J Am Oil Chem Soc, 1991, 68(10)∶ 785-790.
[4]HASNIP S, CREWS C, BRERETON P, et al. A concerted study of factors affecting the formation of 3-MCPD in foods[J]. Pol J Food Nutr, 2002, 52(11)∶ 119-121.
[5]EC Commission Regulation. (EC) No. 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs[S]. Off J Eur Union, L364∶ 5-24.
[6]BRERETON P, KELLY J, CREWS C, et al. Determination of 3-chloro-1,2-propanediol in foods and food ingredients by gas chromatography with mass spectrometric detection∶ collaborative study[J]. Journal of AOAC International, 2001, 84(2)∶ 455-465.
[7]Van BERGEN C A, COLLIER P D, CROMIE D D O, et al. Determination of chloropropanols in protein hydrolysates[J]. J Chromatogr A, 1992, 589(1/2)∶ 109-119.
[8]MATTHEW B M, ANASTASIO C. Determination of halogenated monoalcohols and diols in water by gas chromatography with electron-capture detection[J]. J Chromatogr A, 2000, 866(1)∶ 65-77.
[9]CHUNG W C, HUI K Y, CHENG S C. Sensitive method for the determination of 1,3-dichloropropan-2-ol and 3-chloropropane-1,2-diol in soy sauce by capillary gas chromatography with mass spectrometric detection[J]. J Chromatogr A, 2002, 952(1/2)∶ 185-192.
[10]ABU-EI-HAJ S, BOGUSZ M J, IBRAHIM Z, et al. Rapid and simple determination of chloropropanols (3-MCPD and 1,3-DCP) in food products using isotope dilution GC-MS[J]. Food Control, 2007, 18(1)∶ 81-90. [11]XU Xiaomin, REN Yiping, WU Pinggu, et al. The simultaneous separation and determination of chloropropanols in soy sauce and other flavourings with gas chromatography-mass spectrometry in negative chemical and electron impact ionization modes[J]. Food Addit Contam, 2006, 23(2)∶ 110-119.
[12]CHUNG S W C, KWONG K P, YAU J C W, et al. Chloropropanols levels in foodstuffs marketed in Hong Kong[J]. J Food Comp Anal, 2008, 21(7)∶ 569-573.
[13]LEE M R, CHIU T C, DOU J. Determination of 1,3-dichloro-2-propanol and 3-chloro-1,2-propandiol in soy sauce by headspace derivatization solid-phase microextraction combined with gas chromatography-mass spectrometry[J]. Anal Chim Acta, 2007, 591(2)∶ 167-172.
[14]MARKUS K S, UTE B, ALEXANDRA O G, et al. Rapid and simple micromethod for the simultaneous determination of 3-MCPD and 3-MCPD esters in different foodstuffs[J]. J Agric Food Chem, 2010, 58 (11)∶ 6570-6577.
[15]CAO Xiujun, SONG Guoxin, GAO Yihan, et al. A novel derivatization method coupled with GC-MS for the simultaneous determination of chloropropanols[J]. Chromatographia, 2009, 70(3/4)∶ 661-664.
中图分类号:TS207.3
文献标识码:A
文章编号:1002-6630(2011)14-0232-05
收稿日期:2010-11-09
基金项目:广东省质量技术监督局科技项目(2008SJ029)
作者简介:熊珺(1978—),女,博士,研究方向为痕量化合物的检测技术。E-mail:xxiongjjun@yahoo.com.cn
固相萃取与气相色谱-质谱联用分析调味料中3-氯-1,2-丙二醇
熊 珺,龚 亮,赖毅东
(广东省东莞市质量监督检测中心,广州 东莞 523808)
摘 要:建立固相萃取与气相色谱-质谱联用(solid phase extraction with gas chromatography-mass spectrometry,SPEGC-MS)测定调味料中3-氯-1,2-丙二醇的新方法。对影响分析物SPE萃取效率的诸因素如洗脱溶剂、洗脱溶剂的体积和上样体积等进行详细考察和优化。最佳萃取条件为5.0g样品与5mol/L氯化钠溶液混匀,经SPE萃取净化、衍生后,以GC-MS进行测定,该方法对3-氯-1,2-丙二醇的检出限为0.15μg/kg,线性范围为0.51~6144μg/kg,相关系数和相对标准偏差(relative standard deviation,RSD)(n=5)分别为0.9998和8.8%。该方法成功应用于调味料3-氯-1,2-丙二醇的分析,加标回收的回收率为87.2%~109.4%。
关键词:固相萃取;3-氯-1,2-丙二醇;气相色谱-质谱联用;调味液

