Direct Optical Resolution of Chiral Pesticides by High Performance Liquid Chromatography*
LI Xiaogang (李晓刚)** LIU Yiping (刘一平) HU Changdi (胡昌弟)2, BAI Lianyang(柏连阳)3, GAO Bida (高必达) and HUANG Kelong (黄可龙)4 College of Bio-safety Science & Technology, Hunan Agricultural University, Changsha 4028, China
2 Hunan Institute for the Control of Agrochemicals, Changsha 410005, China
3 Hunan Institute of Humanities, Science and Technology, Loudi 417000, China
4 Institute of Functionalized Materials & Chemistry, Central South University, Changsha 410083, China
1 INTRODUCTION
About 25% of currently used pesticides are chiral,and this ratio is increasing as more compounds with complex structures are introduced into use [1, 2]. Most of chiral pesticides are manufactured and applied to agro-ecosystems as racemic forms, although enantiomers may be different in bioactivity, toxicity, metabolism, bioaccumulation, and degradation behavior.It is essential and urgent to provide chiral separation and enantiomeric analysis methods for chiral pesticides to evaluate the risks to the environment and public health [3-6].
In many chiral resolution techniques, HPLC(High Performance Liquid Chromatography) is a widely used method for the separation and preparation of enantiomers because of its high efficiency and ease of operation, and it is desirable to use chiral stationary phases (CSPs) to separate enantiomers directly. More than 100 CSPs for HPLC have been prepared and commercialized in the past two decades, among which cellulose- and amylose-based CSPs present excellent enantiomeric recognition towards a large number of chiral compounds [7-10]. Polysaccharide CSPs have been primarily used with nonpolar mobile phases since the π-π, dipole-dipole, and hydrogen bond interactions that are believed to be responsible for the chiral identification are more efficient under normal phase conditions. Mobile phase composition, especially the type and the amount of alcohol modifier, is critical to chiral separation by polysaccharide derivative CSPs [11]. Temperature is another factor influencing the selectivity for separation on polysaccharide stationary phases, which should be investigated [12, 13].
Indoxacarb is a member of a new oxadiazine class of insecticides that inhibit sodium ions to enter nerve cells, resulting in the paralysis and death of target insect pests. Indoxacarb is a promising new foliar insecticide against Lepidoptera that attacks vegetables,fruits, vines, cotton, corn and other crops [14]. The indoxacarb molecule has an asymmetric carbon center and two enantiomers, and the insecticidal activity is mainly attributed to the (+)-S-enantiomer [15]. The two enantiomers of indoxacarb can be separated on vancomycin crystalline degradation products [16] and amylose-based [17, 18] CSPs, but no work has been done on cellulose-based CSP. Lambda-cyhalothrin(LCT) is a pyrethroid insecticide, the commercial form of which is mainly used to control mosquitoes,fleas, cock-roaches, flies, etc., and contains only one of the two pairs of its cis-isomers ([Z]-1R-cis-αS and[Z]-1S-cis-αR, 1∶1). It was separated with polysaccharide-based CSPs columns and the aquatic toxicity of two enatiomers against vertebrate zebrafish and their embryos was evaluated [19]. Simeconazole was introduced by Sankyo Agro Co. Ltd as a highly active,broad spectrum trizole fungicide for the control of rice sheath blight caused byRhizoctonia solaniKuhn in paddy water [20, 21], with a chemical name of[2-(4-fluorophenyl)-1-(1H-1,2,4-triazol-1-yl)-3-trimet hylsilylpropan-2-ol] and two enantiomers due to the asymmetric carbon atom. The two enantiomers of simeconazole were separated by HPLC on amylosetris[(s)-α-methyphenyl-carbamate] CSP [22]. The chemical structures of the three chiral pesticides are shown in Fig. 1.
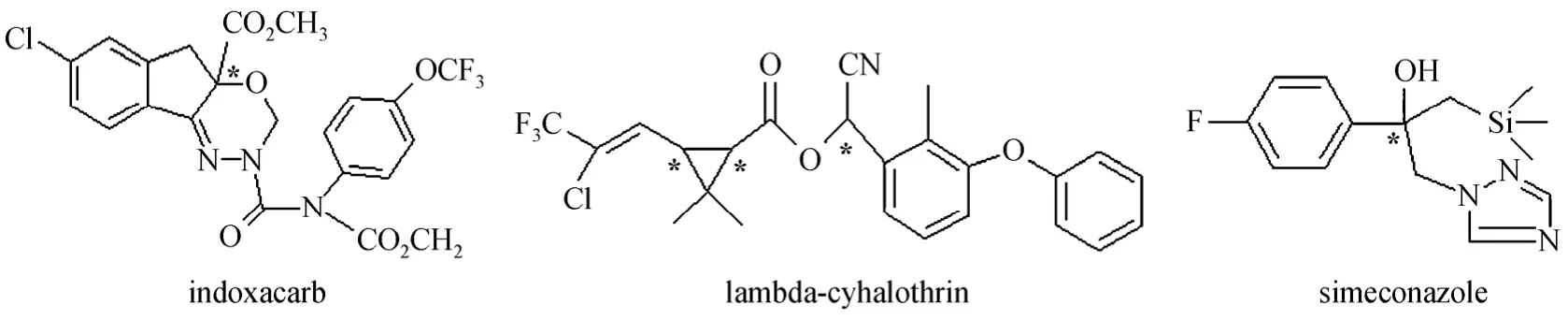
Figure 1 Chemical structures of the three chiral pesticides*: chiral center
In this work, three chiral pesticides are directly separated by HPLC on cellulose tris-(3,5-dimethylphenylcarbamate)-coated chiral stationary phase CDMPCCSP under normal phase conditions. The influences of five alcohol modifiers, flow rate and temperature from 15 to 35 °C on the separations are investigated. The thermodynamical mechanism of enantioseparation and chiral recognition mechanism are also discussed.
2 MATERIALS AND METHODS
2.1 Chemicals and reagents
Analytical standard of racemic indoxacarb (97%)and lambda-cyhalothrin (95%) was provided by National Engineering Research Center for Agrochemicals,Hunan Research Institute of Chemical Industry. Racemic simeconazole (98%) was obtained from Hunan Institute for the Control of Agrochemicals. All eluents were chromatographic grade.n-hexane (95%),n-propanol(99%),iso-propanol (99.9%), andn-butanol (99%)were purchased from J&K SCIENTIFIC Ltd., anhydrous ethanol (90%) andiso-butanol (99%) were purchased from Alfa Aesar China (Tianjin) Co., Ltd. The mobile phase eluents were distilled before use.
2.2 Apparatus
Chromatography was performed using an SHIMADZU LC-20AT HPLC (SHIMADZU Corporation,Japan) equipped with LC-20AT pumps, a 20 μl sample loop and UV-VIS Detector. The signal was acquired and processed by LC-solution workstation.
2.3 Chromatographic conditions
The commercial HPLC column Chiralcel OD-H[cellulose tris-(3,5-dimethylphenyl-carbamate)] was purchased from Daicel Chiral Technologies (China)CO., LTD, and it was 250×4.6 mm (i.d.). The mobile phase wasn-hexane with an appropriate percentage of ethanol,n-propanol,iso-propanol,n-butanol oriso-butanol as modifier. Chromatographic resolutions were performed at room temperature except for the experiment to investigate the influence of temperature,which was performed over a range of 15-35°C with 15%, 10%, and 15%iso-propanol inn-hexane for indoxacarb, lambda-cyhalothrin and simeconazole. The flow rate was 1.0 ml·min-1except in the experiment for the effect of flow rate. The samples were injected with amounts of 20 μl, and the detection wavelength was 254 nm. The capacity factor (k′) was determined ask′ = (t-t0) /t0. The enantioselectivity factor (α)was calculated asα= (k2′ /k1′), wherek1′ andk2′ are retention factors for the first and second eluting enantiomers, respectively. The resolution (Rs) was determined asRs= 2 (t2-t1)/(w1+w2), wherew1andw2are base widths for the first and second eluting enantiomers, respectively. The void time (t0) was determined using 1,3,5-tri-tert-butylbenzene.
3 RESULTS AND DISCUSSION
3.1 Influence of mobile phase composition
Mobile phase plays the most important role for enantiomeric separations in terms of efficiency, retention, and resolution of enantiomers. Therefore, the composition of mobile phase should be investigated in the optimization of enantioselectivity [23-25]. The chiral separation was performed usingn-hexane-polar organic alcohols as mobile phase in this work. The effects of five alcohols, ethanol,n-propanol,iso-propanol,n-butanol, andiso-butanol, and their volume content in the mobile phase on the resolution were investigated.
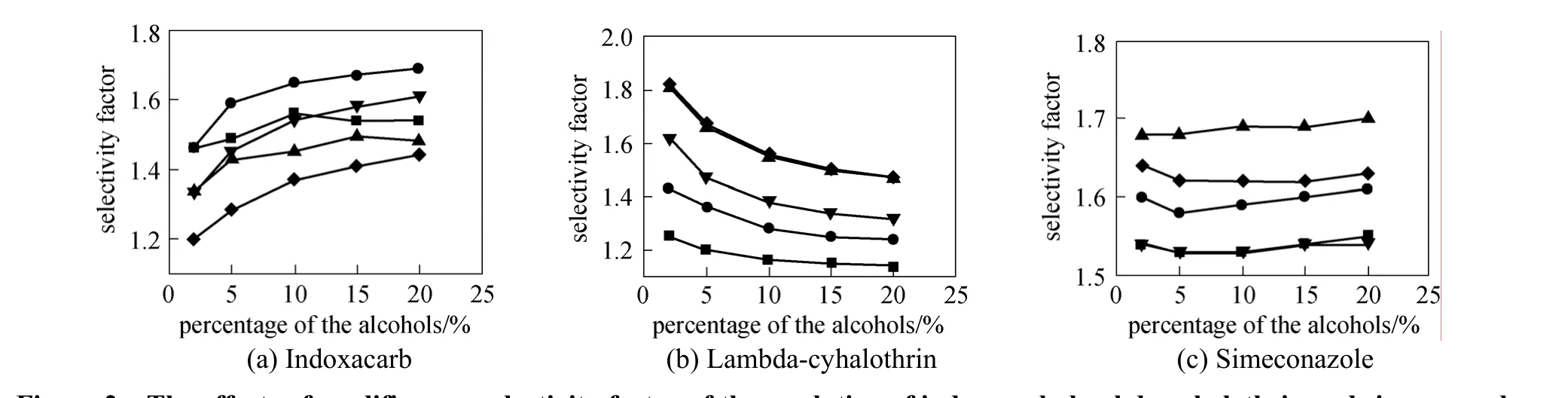
Figure 2 The effects of modifiers on selectivity factor of the resolution of indoxacarb, lambda-cyhalothrin and simeconazole■ ethanol; ● n-propanol; ▲ iso-propanol; ▼ n-butanol; ◆ iso-butanol
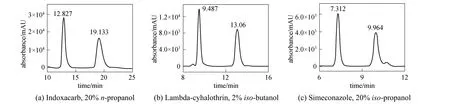
Figure 3 Chromatograms of chiral separation of the three chiral pesticides(column temperature 25 °C, flow rate 1.0 ml·min-1, inject volume 20 μl, wavelength 254 nm)
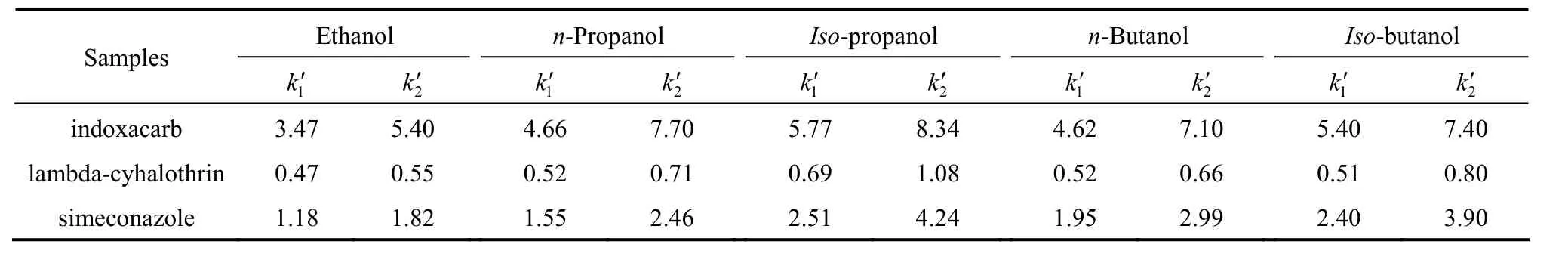
Table 1 The effect of modifiers on the retention in n-hexane mobile phase(10% of modifier in n-hexane, column temperature 25 °C, flow rate1.0 ml·min-1)
Figure 2 shows the effects of five modifiers on the selectivity factor (α) of the resolution of three chiral pesticides inn-hexane at 25 °C. For separating the two enantiomers of indoxacarb [Fig. 2 (a)], the effect of modifiers isiso-butanol<iso-propanol<n-butanol<ethanol<n-propanol. Five modifiers all give complete resolution for the two enantiomers, andn-propanol shows higher stereoselectivity. The highestαvalue is 1.69 when the volume percentage ofn-propanol is 20%. For the separation of lambdacyhalothrin enantiomers [Fig. 2 (b)], the selectivity factor decreases as the modifier percentage increases in the mobile phase, and the best resolution efficiency of lambda-cyhalothrin is achieved when the concentration of modifiers is 2%. The effect of modifiers isiso-butanol>iso-propanol>n-butanol>n-propanol>etha nol.Iso-butanol,iso-propanol andn-butanol give separation at any volume percentage, and the best resolution of lambda-cyhalothrin is achieved using 2%iso-butanol withαvalue of 1.82. Ethanol is the worst modifier for the separation of lambda-cyhalothrin. For separation of simeconazole [Fig. 2 (c)], all the five alcohols give baseline separation at any volume percentage from 2% to 20%, among whichiso-propanol presents more advantages for the separation compared with the other modifiers. The highestαvalue is 1.70 when the percentage ofiso-propanol is 20%. Fig. 3 shows the chromatograms of the resolutions of three analyte at room temperature.
Table 1 lists the effects of different modifiers(10% inn-hexane mobile phase) on the capacity factors of the two enantiomers of the three chiral pesticides on the CSP. The retention of the samples on the CSP in the descending order is indoxacarb>simeconazole>lambda-cyhalothrin. The samples show the strongest retention usingiso-propanol modifier, followed byiso-butanol,n-propanol,n-butanol, and ethanol.
The results indicate that the modifiers compete with the solutes for the interactions with the CSP. The polarities and viscosities of the alcohols may not be the only factors influencing the chiral separation, and the structure of alcohols have some effect on the stereoselectivity, perhaps by altering the environment of the chiral cavity [26].
3.2 Influence of flow rate
The effect of flow rate on enantioseparation was investigated with the hexane as mobile phase and 15%iso-propanol as modifier at room temperature. Fig. 4 shows the effects of flow rate on enantiomeric separation of enantiomers of indoxacarb, lambda-cyhalothrin and simeconazole. No significant change appears in selectivity factor (α), but the resolution (Rs) decreases as the flow rate increases in the range of 0.4-1.1 ml·min-1.Lower flow rate is preferred for enantioseparation, but it causes longer retention time and peak trailing.
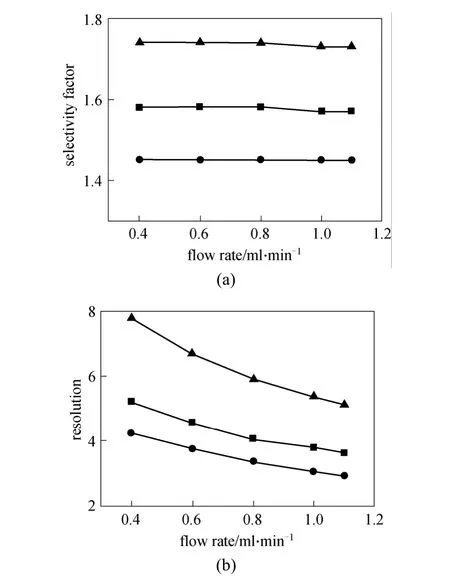
Figure 4 Influence of e flow rate on the selectivity factor and resolution(mobile phase: n-hexane/iso-propanol=85∶15, column temperature 25°C )■ indoxacarb; ● lambda-cyhalothrin; ▲ simeconazole
3.3 Influence of temperature on separation
Column temperature is an important factor for enantiomer separation [27-29]. In this study, the enantioselective separation of three chiral compounds was investigated with stepwise increase of column temperature from 15-35 °C with 5 °C increment, and the thermodynamical mechanism of enantioseparation is discussed. The calculated parameters including retention factor (k′), selectivity factor (α) and resolution(Rs) are summarized in Table 2. Generally, an increase in column temperature often causes a reduction in retention and worse resolution. The effect of temperature on the resolution of lambda-cyhalothrin is examined usingn-hexane/iso-propanol 90∶10 as mobile phase. The capacity factor (k′), separation factor (α)and the resolution factor (Rs) decrease as temperature increases.αandRsshow the maximum values of 1.54 and 4.04 at 15 °C, respectively. The mobile phase ofn-hexane/iso-propanol 85∶15 is adopted for investigation of the effect of temperature on the separation of indoxacarb and simeconazole. The values ofk′ andαgradually decrease with increasing temperature, butRsdoes not change regularly with temperature with the peak broadening at low temperature.Rsreaches the highest values 3.97 and 5.48 at 35°C since the peak trails seriously at lower temperature.
As a crucial factor, temperature affects chiral separation in at least two ways [30]. One is kinetic effect that influences the viscosity and diffusion coefficient of the solute. Another is thermodynamic effect that changes the separation factor. The separation efficiency usually decreases with increasing temperature,which may be due to the decrease of change of Gibbs free energy (ΔR,SΔGө=-RTlnα) for transfer of analyte between stationary phase and mobile phase at high temperature.
The enthalpic and entropic contributions to enantioselectivity may be described using the following vant’t Hoff equations:
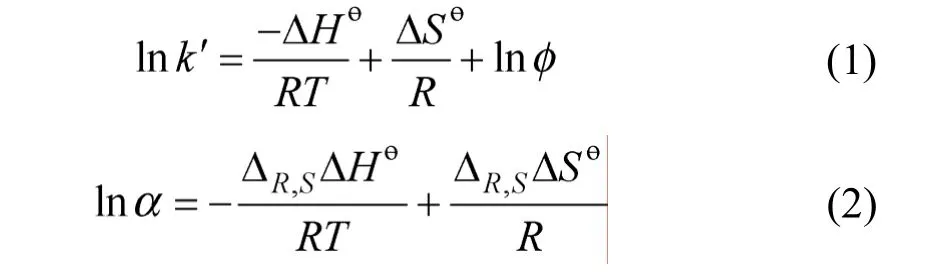
wherek′ is a retention factor,Ris the gas constant,Tis the absolute temperature, ΔHөand ΔSөare the standard transfer enthalpy and entropy of analyte from mobile phase to stationary phase,φis the phase ratio,ΔR,SΔHө= ΔH2-ΔH1and ΔR,SΔSө= ΔS2-ΔS1. Eq. (1)indicates that if the plot of lnk′versus1/Tis linear, the slope and intercept are -ΔHө/Rand ΔSө/R+ lnφ, respectively. For a linear plot of lnαversus1/T, the slope and intercept are -ΔR,SΔHө/Rand ΔR,SΔSө/R, respectively. At isoelution temperature (Tiso), the enthalpic and entropic contributions to chiral recognition are balanced and the enantiomers co-elute [31]. AtTiso,lna=0, and ΔR,SΔHө/RT= ΔR,SΔSө/R, solving for 1/T:
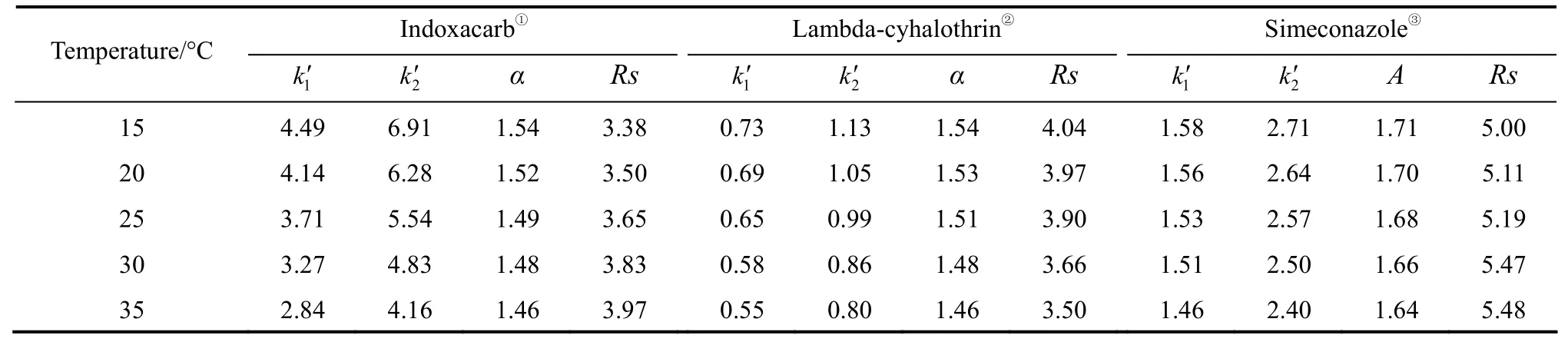
Table 2 The effect of temperature on chiral resolution of three chiral samples (flow rate 1.0 ml·min-1)
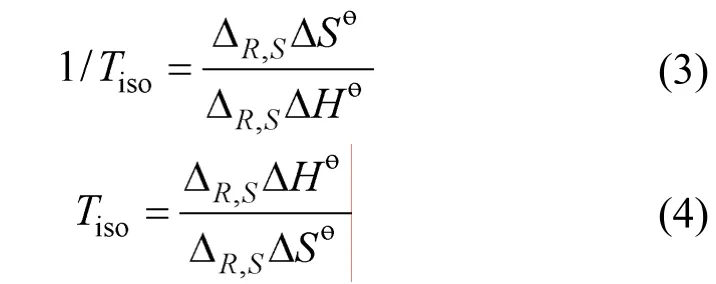
At this temperature, elution order of the enantiomers will change, and chiral recognition is said to be enthalpy controlled. Above this temperature, elution order of the two enantiomers is controlled by entropy.Fig. 5 shows the linear vant’t Hoff plots of simeconazole from 15 to 35°C, in which lnk′ and lnαversus1/Tfor simeconazole are both linear in the mobile phase hexane/iso-propanol and the selectivity factor (α) decreases as temperature increases. The vant’t Hoff linear equations (R>0.98) and thermodynamical parameters for the chiral pesticides are given in Table 3. For all the samples, enthalpic terms are negative, indicating that the association process between the three analytes and stationary phase is enthalpically favored.Low temperature is better for the resolutions.
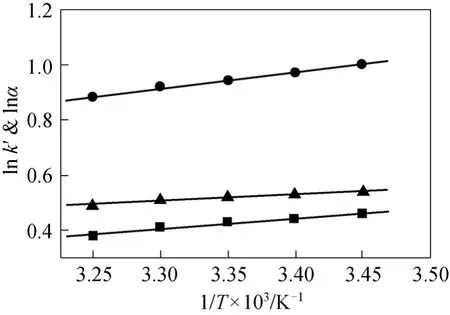
Figure 5 The vant’t Hoff plot for k′ and α for the enantiomers of simeconazole with n-hexane/iso-propanol (volume ratio: 85∶15, flow rate: 1.0 ml·min-1)■ 1lnk′; ● 2lnk′; ▲ lnα
The complex process for distinguish enantiomers may be affected by other factors. Although many studies on the effect of temperature on chiral separation were performed, the mechanism of temperature effect on the enantioselectivity is not explicitly explained, especially the way the temperature alters the enthalpy and entropy change related to the transfer of solutes from mobile phase to stationary phase [32].
3.4 Separation mechanism
It is commonly considered that chiral separation by CSPs is achieved through hydrogen, π-π, and dipole-dipole induced interactions between the analyte enantiomers and polar carbamate groups of polysaccharide tris(phenylcarbamate) CSP [33]. The structures of analytes for resolutions have an electronegative atom(nitrogen, oxygen, or sulfur), C O group, or phenyl ring directly attaching to the chiral center, which may interact with the CSP through hydrogen bonding, dipole-dipole, or π-π interactions. Indoxacarb has C O,chlorine atoms and phenyl groups that can interact with the polar carbamate groups mainlyvia(1) hydrogen bonding with C O, chlorine and NH2groups,(2) dipole-dipole interaction between C O groups and (3) π-π interactions between aromatic rings.Lambda-cyhalothrin contains chlorine, fluorine electronegative atoms and cyanogen group attaching to chiral center, which may interact with the amino group of CSP by strong hydrogen bonding interaction. Simeconazole does not have C O group, so dipole-dipole interaction may not occur between analytes and CSP,hydrogen bonding and π-π interactions may cause the chiral resolutions. Different interactions of enantiomers and CSP contribute to the chiral identification.In addition, the size and the geometric configuration of the solutes may be significant factors [34].
4 CONCLUTIONS
Three chiral pesticides were directly separated by HPLC on the CDMPC-CSP (Chialcel OD-H column)under normal phase conditions. It demonstrates thatChiralcel OD-H is an efficient technique for separating racemic indoxacarb, lambda-cyhalothrin and simeconazole. The HPLC method is a simple, rapid and effective way to separate the enantiomers of the three chiral compounds, investigate the chiral discriminating mechanism, and determine the target enantiomers quantitatively in ecosystem for further study in tracing different bioactivities, metabolism and environmental behavior, minimizing the risks from chiral pesticides to the environment and public health [35].
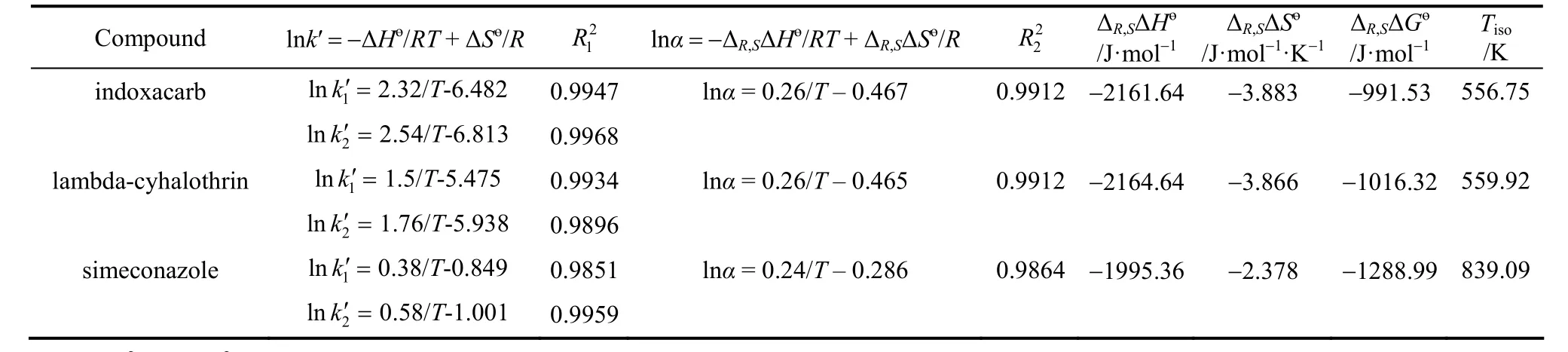
Table 3 The vant’t Hoff equations and the thermodynamic parameters
NOMENCLATURE

1 Liu, W.P., Gan, J.Y., Schlenk, D., Jury, W.A., “Enantioselectivity in environmental safety of current chiral insecticides”, Proc. Natl. Acad.Sci. U.S.A., 102 (3), 701-706 (2005).
2 Li, Z.Y., Wu, T., Li, Q.L., Zhang, B.Z., Wang, W.X., Li, J.Y., “Characterization of racemization of chiral pesticides in organic solvents and water”, J. Chromatogr. A., 1217 (36), 5718-5723 (2010).
3 Peng, X., Liu, D.H., Diao, J.L., Lu, D.H., Zhou, Z.Q., “Enantioselective acute toxicity and bioaccumulation of benalaxyl in earthworm (Eisenia fedtia)”, J. Agric. Food Chem., 57 (18), 8545-8549(2009).
4 Li, L., Zhou, S.S., Jin, L.X., Zhang, C., Liu, W.P., “Enantiomeric separation of organophosphorus pesticides by high-performance liquid chromatography, gas chromatography and capillary electrophoresis and their applications to environmental fate and toxicity assays”, J. Chromatogr. B., 878 (17-18), 1264-1276 (2010).
5 Sun, J.Q., Liu, J.S., Tu, W.Q., Xu, C., “Separation and aquatic toxicity of enantiomers of the organophosphorus insecticide O-ethyl O-4-nitrophenyl phenylphosphonothioate (EPN)”, Chemosphere, 81(10), 1308-1313 (2010).
6 Xu, P., Diao, J.L., Liu, D.H., Zhou, Z.Q., “Enantioselective bioaccumulation and toxic effects of metalaxyl in earthworm Eisenia foetida”, Chemosphere, 83 (8), 1074-1079 (2011).
7 Yashima, E., “Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation”, J.Chromatogr. A., 906 (1-2), 105-125 (2001).
8 Aboul-Enein, H.Y., “High-performance liquid chromatographic enantioseparation of drugs containing multiple chiral centers on polysaccharide-type chiral stationary phases”, J. Chromatogr. A., 906(1-2), 185-193 (2001).
9 Okamoto, Y., Ikai, T., “Chiral HPLC for efficient resoluton of enantiomers”, Chem. Soc. Rev., 37 (12), 2593-2608 (2008).
10 Okamoto, Y., “Chiral Polymers for Resolution of Enantiomers”, J.Polym. Sci. Part A: Polym. Chem., 47 (7), 1731-1739 (2009).
11 Wang, T., Wenslow, R.M.J., “Effects of alcohol mobile phase modifiers on the structure and chiral selectivity of amylose tris(3,5-dimethylphenylcarbamate) chiral stationary phase”, J. Chromatogr.A., 1015 (1-2), 99-110 (2003).
12 Soonkoo, H., Kyungho, R., “Chiral separation of Ibuprofen by supercritical fluid chromatography”, Chin. J. Chem. Eng., 13 (6),741-746 (2005).
13 Peter, A., Vekes, E., Armstrong, D.W., “Effects of temperature on retention of chiral compounds on a ristocetin A chiral stationary phase”, J. Chromatogr. A., 958 (1-2), 89-107 (2002).
14 Wing, K.D., Sacher, M., Kagaya, Y., Tsurubuchi, Y., Mulderig, L.,Connair, M., Schnee, M., “Bioactivation and mode of action of the oxadiazine indoxacarb in insects”, Crop Protection, 19 (8-10),537-545 (2000).
15 Wing, K.D., Schnee, M.E., Sacher, M., Connair, M., “A novel oxadiazine insecticide is bioactivited in Lepidopteran larvae”, Archives of insect biochemistry and physiology, 37 (2), 91-103 (1998).
16 Mohammad, M.M., Chalavi, S., Ghassempour, A., Tabar-Heydar, K.,Sharif, S.J.G., Malekzadeh, M., Aboul-Enein, H.Y., “Chiral separation of three agrochemical toxins enantiomers by high-performance liquid chromatography on a vancomycin crystalline degradation products-chiral stationary phase”, Biomed. Chromatogr., 21 (3),234-240 (2007).
17 Saito, K., Yato, M., Ito, T., Iwasaki, Y., Ito, R., Matsuki, Y., Nakazawa, H., “Verification of the need for optical purity measurement of chiral pesticide standards as agricultural reference materials”, Accred.Qual. Assur., 13 (7), 373-379 (2008).
18 Dong, F.S., Cao, Q., Liu, X.G., Zheng, Y.Q., Li, C.J., “Enantiomeric separation of indoxacarb by HPLC with amylose chiral column”,Chemical Regents, 30 (7), 517-518; 521 (2008). (in Chinese)
19 Xu, C., Wang, J.J., Liu, W.P., Daniel, S.G., Tu, Y.J., Ma, Y., “Separaiton and aquatic toxicity of enantiomers of the pyrethroid insecticide lambda-cyhalothrin”, Environmental Toxicology and Chemistry,27 (1), 174-181 (2008).
20 Tsuda, M., Kato, S., “Effects of soil adsorption on uptake of simeconazole by rice plants and sheath blight control effect”, Annual Report of the Society of Plant Protection of North Japan, 54, 32-34(2003).
21 Tsuda, M., Itoh, H., Kato, S. “Systemic activity of simeconazole and its derivatives in plants”, Pest Management Science, 60 (9), 881-886(2004).
22 Dong, F.S., Cao, Q., Liu, X.G., Zheng, Y.Q., Li, C.J., “Enantiomeric separation of simeconazole by HPLC with amylose chiral column”,Chinese Journal of Applied Chemistry, 25 (10), 1237-1239 (2008).(in Chinese)
23 Liu, D.H., Wang, P., Zhou, W.F., Gu, X., Chen, Z.S., Zhou, Z.Q.,“Direct chiral resolution and its application to the determination of fungicide benalaxyl in soil and water by high-performance liquid chromatography”, Analytica Chimica Acta, 555 (2), 210-216 (2006).
24 Wang, P., Liu, D.H., Jiang, S.R., Gu, X., Zhou, Z.Q. “The direct separation of fungicide enantiomers on amylopectin based chiral stationary phase by HPLC”, Chirality, 19 (2), 114-119 (2007).
25 Tan, X.P., Hou, S.C., Wang, M., “Enantioselective and diastereoselective separation of synthetic pyrethroid insecticides on a novel chiral stationary phase by high-performance liquid chromatography”,Chirality, 19 (7), 574-580 (2007).
26 Wang, P., Jiang, S.R., Liu, D.H., Wang, P., Zhou, Z.Q., “Direct enantiomeric resolutions of chiral triazole pesticides by highperformance liquid chromatography”, J. Biochem. Biophys. Methods,62 (3), 219-230 (2005).
27 Zhou, Y., Li, L., Lin, K.D., Zhu, X.P., Liu, W.P., “Enantiomer separation of triazole fungicides by high-performance liquid chromatography”, Chirality, 21 (4), 421-427 (2009).
28 Rao, R.N., Nagaraju, D., Raju, A.N., “Enantiomeric resolution of doxazosin mesylate and its process-related substances on polysaccharide chiral stationary phases”, J. Pharmaceut. Biomed., 41 (3),766-773 (2006).
29 Blackwell, J., Stringham, R.W., “Temperature effects for chiral separation using various bulk fluids in near-critical mobile phase”,Chirality, 9 (7), 693-698 (1997).
30 Lin, K.D., Xu, C., Zhou, S.S., Liu, W.P., Gan, J., “Enantiomeric separation of imidazolinone herbicides using chiral high-performance liquid chromatography”, Chirality, 19 (3), 171-178 (2007).
31 Stringham, R.W., Blackwell, J.A., “Factors that control successful entropically driven chiral separations in SFC and HPLC”, Anal.Chem., 69 (7), 1414-1420 (1997).
32 Wang, P., Jiang, S.R., Liu, D.H., Zhang, H.J., Zhou, Z.Q., “Enantiomeric resolution of chiral pesticide by high-performance liquid chromatography”, J. Agric. Food. Chem., 54 (5), 1577-1583 (2006).
33 Yamamoto, C., Yashima, E., Okamoto, Y., “Computational studies on chiral discrimination mechanism of phenylcarbamate derivatives of cellulose”, Bull. Chem. Soc. Jpn., 72 (8), 1815-1825 (1999).
34 Wang, P., Jiang, S.R., Liu, D.H., Jia, G.F., Wang, Q.X., Wang, P.,Zhou, Z.Q., “Effect of alcohols and temperature on the direct chiral resolutions of fipronil, isocarbophos and carfentrazone-ethyl”,Biomedical chromatography., 19 (6), 454-458 (2005).
35 Ye, J., Zhao, M.R., Liu, J., Liu, W.P., “Enantioselectivity in environmental risk assessment of modern chiral pesticides”, Environmental Pollution, 158 (7), 2371-2383 (2010).
 Chinese Journal of Chemical Engineering2011年4期
Chinese Journal of Chemical Engineering2011年4期
- Chinese Journal of Chemical Engineering的其它文章
- Synthesis of Methyl Isopropyl Ketone and Diethyl Ketone over Ni-Na/ZrO2-MnO2-ZnO Catalyst*
- Isotherm Equation Study of F Adsorbed from Water Solution by Fe2(SO4)3-modified Granular Activated Alumina*
- Preparation and Dyeing Performance of a Novel Crosslinking Polymeric Dye Containing Flavone Moiety*
- Solubility of Paclitaxel in Mixtures of Dichloromethane and Supercritical Carbon Dioxide*
- The Kinetics for Electrochemical Removal of Ammonia in Coking Wastewater*
- Solvent Recovery from Soybean Oil/Hexane Miscella by PDMS Composite Membrane*
