共表达H5N1流感病毒M1和HA基因的DNA疫苗与腺病毒载体疫苗的小鼠免疫评价
郭建强,姚立红,陈爱珺,刘晓宇,付金奇,徐鹏卫,张智清
中国疾病预防控制中心病毒病预防控制所 病毒基因工程国家重点实验室,北京 100052
Introduction
The emergence of highly pathogenic avian influenza virus (HPAIV) strains has resulted in huge economic losses, and it poses a serious threat of increased patient morbidity and mortality. The first case of a human patient infected with H5N1 avian influenza virus (AIV) was reported in Hong Kong in 1997[1-2]. In 2005, the first human infection of H5N1 AIV in China was reported in Anhui Province, from which the A/Anhui/1/2005 virus strain was isolated[3]. Since then, the number of human cases of H5N1 AIV infection has been increasing. If the virus were to mutate and gain the ability to spread directly from human to human, an influenza pandemic would be inevitable. Given the urgency of this potential pandemic, the development of a safe and effective vaccine that is both simple to produce and suitable for use in humans is a critical task.
Hemagglutinin (HA) is a glycoprotein embedded on the influenza virus particle surface that has the ability to bind to receptors on target cells and agglutinate red blood cells. HA plays an important role in the process of viral attachment and fusion to the membrane of target cells. HA also induces protective neutralizing antibodies and is, therefore, an important component of influenza vaccines. However, due to the low conserved protein sequence, the HA based seasonal influenza vaccine currently widely used cannot provide efficient protection against H5N1 virus. Matrix protein 1 (M1) is the most abundant protein in virus particle and is type-specific. M1 is also multi-functional, playing important roles in the regulation of viral replication. These roles[4-6]include controlling the transcription of viral genes and the transport of substances between the nucleus and cytoplasm of infected cells, adjusting progeny virus particle assembly and stabilizing the core of virus particles by interacting with the trans-membrane domain of HA, neuraminidase (NA) and matrix protein 2 (M2).
DNA vaccine is very safe. It can effectively stimulate humoral and cellular immune responses and is therefore suitable for the first inoculation in a combined immunization regime. With a strong CMV promoter and internal ribosome entry site (IRES) sequence, plasmid pStar can be used to build dual-gene co-expression DNA vaccines. Adenovirus vectors can highly express exogenous proteins in mammalian cells and have been widely used in vaccine research. Non-replicable adenovirus represents a safer alternative to replicable adenovirus. The generation of low dose of protein antigen and the failure to lyse cells are two features that are helpful in stimulating long-term immune responses[7]. Recombinant adenoviral vaccines expressing influenza virus HA protein have been previously reported[8-9].
In a previous study, we constructed a recombinant plasmid, pStar-M1/HA, using the pStar plasmid containing the IRES sequence, and a recombinant adenovirus, Ad-M1/HA, by inserting an IRES to link the M1 and HA genes. In this study, pStar-M1/HA and Ad-M1/HA were used to immunize BALB/c mice with a combined prime-boost regime and the immune responses of the mice were evaluated.
1 Materials and methods
1.1 Plasmids, strains, cells, animals and reagents
Plasmid pStar was a gift from Dr. Yiyou Chen, (Starvax Co. in Beijing, China). pStar-M1/HA, pStar-HA, Ad-M1/HA, Ad-HA and Ad-easy (containing no exogenous gene) were constructed in this lab. Inactivated H5N1 influenza virus (A/Anhui/1/2005), M1 and HA genes of this virus strain, receptordestroying enzyme, horse red blood cells (HRC) and turkey red blood cells (TRC) were provided by Chinese Influenza Center. 5-week-old female BALB/c mice were purchased from Beijing Vital River Laboratory Animal Technology. Restriction enzymes and EndoFree Plasmid Maxi Kit were purchased from Qiagen. DMEM, FBS, OPTI-MEM and lipofectamine 2000 were purchased from Invitrogen. Rabbit Anti-M1 (H5N1) polyclonal antibody, mouse anti-HA (H5N1) polyclonal antibody and anti H5N1 influenza virus polyclonal antibody were prepared in this lab. FITC-labeled goat anti-rabbit IgG, FITC-labeled goat anti-mouse IgG and HRP labeled goat anti-mouse IgG were purchased from Zhongshan Goldbridge Biotechnology. Western blotting chemiluminescence detection kit was purchased from Promega. CpG motifs (5′-TCCATGACGTTCCTGACGTT-3′) added into DNA vaccines as adjuvant were synthesized by TaKaRa, and provided by Dr. Jiang Tao. HA peptide library (77 peptides, 16−18 mer, overlapping by 10 amino acids) representing the entire HA (H5N1) protein sequence and M1 peptide library (31 peptides) representing the entire M1(H5N1) protein sequence were gifts from Dr. Chris Li, (Medical Research Council, UK), and were synthesized by Sigma. Lymphocyte separation solution EZ-SepTMwas purchased from Dakewe Biotech. ELISPOT Kits were purchased from BD.
1.2 Amplification and detection of pStar-M1/HA
Recombinant Escherichia coli DH5α strain containing pStar-M1/HA was amplified and the plasmid was extracted using the EndoFree Plasmid Maxi Kit. The concentration of recombinant plasmid DNA was determined based on the optical density at 260 nm (OD260). Successful insertion of the M1 and HA genes was confirmed using restriction enzyme digestion. Twenty-four hours after transfection of 293 cells with the plasmid, M1 and HA gene expression was detected by an indirect immunofluorescence assay (IFA) using rabbit anti-M1 and mouse anti-HA polyclonal antibodies. pStar and pStar-HA were also prepared as control.
1.3 Preparation of recombinant adenovirus Ad-M1/HA
HEK-293 cells at a low passage number were cultured and expanded from 10 mm cell culture dishes to multiple roller bottles. 293 cells were infected with adenovirus at a MOI of 103virus particles/cell. Forty-eight to seventy-two hours after infection, the cells were collected and resuspended in 10 mmol/L Tris·HCl, centrifuged at 12 000 ×g for 5 min. The supernatant was collected and filtered through a 0.22 µm filter. The adenovirus was further purified using QXL column chromatography. Ad-HA and Ad-easy were also prepared as control.
1.4 Titration of recombinant adenovirus
Infectious viral titers were determined using the TCID50on HEK-293 cells and the concentration of viral particles (VP/mL) was determined based on the OD260. Viral purity was based on a determination of the OD260/280ratio, which was expected to be 1.2−1.4 after purification. The insertion of the M1 and HA genes was confirmed by PCR, using Ad-M1/HA virus DNA as template and three pairs of primers: 1) upstream primer of the M1 gene and downstream primer of the HA gene; 2) upstream and downstream primers of the HA gene; 3) upstream and downstream primers of M1 gene. Co-expression of M1 and HA genes was confirmed by IFA and Western blotting after infection of 293 cells with the recombinant adenovirus.
1.5 Animal experiments
5-week-old female BALB/c mice were used for in vivo studies. The mice were immunized with a combination of the DNA and adenoviral vaccines, which were delivered via intramuscular injection in a prime-boost regime. Mice were randomly assigned into one of 3 groups (five mice per group): group M1HA was immunized with pStar-M1/HA and Ad-M1/HA, group HA was immunized with pStar-HA and Ad-HA and group NC was immunized with pStar and Ad-easy. At days 0 and 28, the mice were immunized with the DNA vaccine together with a CpG adjuvant (100 µg plasmid DNA plus 10 µg CpG per mouse). At days 14 and 42, the mice were immunized with the recombinant adenovirus (2.5×108TCID50per mouse).
1.6 Evaluation of the immune response in mice
Before each injection, and 14 days after the final injection, blood samples were collected by bleeding from the saphenous vein for hemagglutination inhibition (HI) assay and enzyme-linked immunosorbent assay (ELISA). The sera were treated with receptor-destroying enzyme before being tested for H5-specific antibodies in HI assay. The assay was performed on HA plate using 4 hemagglutination units of inactivated H5N1 influenza virus and 1% fresh TRC or HRC. The mice sera were diluted serially and added to the wells. HI titers were determined based on the highest dilution of serum inhibiting agglutination of 1% red cells.
H5N1 influenza virus-specific immunoglobulin G (IgG) antibodies were detected by ELISA using either inactivated H5N1 influenza virus or recombinant M1 (H5N1) protein to coat plates. ELISA results were determined by ELISA reader at OD450.
At day 56, the mice were sacrificed and splenocytes were collected and examined by enzyme-linked immunospot (ELISPOT) assay. Splenocytes were plated at 2×105cells per well and individual synthetic HA or M1 peptide fragments of H5N1 influenza virus were use to detect IFN-γ-secreting cells.
2 Results
2.1 Preparation and identification of pStar-M1/HA and recombinant adenovirus Ad-M1/HA
pStar-M1/HA was extracted using the EndoFree Plasmid Maxi Kit and successful insertion of the M1 and HA genes was confirmed using restriction enzyme digestion. After transfection of 293 cells with the plasmid, co-expression of M1 and HA was confirmed by IFA using rabbit anti-M1 and mouse anti-HA polyclonal antibodies (Fig. 1A). pStar-HA and pStar were also prepared and identified as described above.
2.2 Preparation of recombinant adenovirus Ad-M1/HA
Recombinant adenoviruses, Ad-M1/HA, Ad-HA, and Ad-Easy were amplified in HEK-293 cells and purified by QXL column chromatography. As shown on table 1, infectious viral titers were around 1.0%−1.3% (TCID50/VP) and the concentration of viral particles was around 1.0×1012(VP/mL). Adenovirus purity determined based on the OD260/OD280ratio was about 1.20, indicating the virus quality is good for in vivo study.
2.3 Identification of recombinant adenovirus
Twenty four hours after infection of 293 cells with Ad-M1/HA, expression of the M1 and HA genes was detected by IFA using rabbit-anti M1 and mouse-anti HA polyclonal antibodies. Yellow-green fluorescentwere observed in 293 cells infected with Ad-M1/HA, no fluorescence was observed in 293 cells infected with Ad-easy (Fig. 1B). These results confirmed that both the M1 and HA genes inserted into the adenovirus Ad-M1/HA genome were expressed successfully. When the cells infected with Ad-M1/HA showed a cytopathic effect (CPE) of more than 50%, the cells were collected, analyzed by Western blotting with a mouse anti-influenza virus (H5N1) polyclonal antibody. The blots identified bands of 29 kDa and 50 kDa, which corresponded to the M1 and HA1 proteins, respectively. This result confirmed that the M1 and HA genes were co-expressed successfully (Fig. 1C).
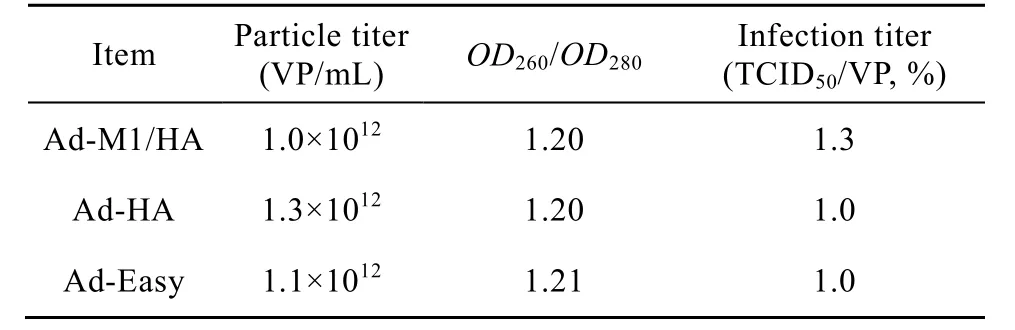
Table 1 Parameter of recombinant adenovirus after purification

Fig. 1 Co-expression of M1 and HA genes. (A) Co-expression of M1 and HA genes on pStar-M1/HA detected by IFA (200×). 1: detection of M1 protein; 2: detection of HA protein. (B) co-expression of M1 and HA genes on Ad-M1/HA detected by IFA (200×). 1: detection of M1 protein; 2: detection of HA protein. (C) co-expression of M1 and HA genes of Ad-M1/HA detected by Western blotting. 1: protein marker; 2: 293 cells infected with Ad-M1/HA.
2.4 Detection of HI activity in mouse sera
DNA vaccines and adenoviral vaccines were combined to immunize BALB/c mice with a prime-boost regime and blood samples were collected for HI assay using TRC. HI activity could be detected in sera of the M1HA group 14 days after the second immunization and the HI titer gradually increased with the subsequent boosting (P<0.05) (Fig. 2). There were no significant differences (P>0.05) between the HI titers of M1HA group and HA group. No HI activity could be detected in the sera of NC group. Furthermore, a comparison of HRC and TRC indicated that both of them demonstrated HI activity in the sera of immunized mice and that HRC provided a higher sensitivity than TRC (Fig. 3).
Fig. 2 HI activity increased with the subsequent boosting of immunization. Before each injection and 14 days after the final injection, blood samples were collected and tested for HI antibody against H5N1 influenza virus. HI titers were determined based on the highest dilution of each serum sample inhibiting agglutination of 1% TRC by four HA units of inactivated H5N1 influenza virus. HI activity could be detected in sera 14 days after the second immunization and gradually increased with the subsequent boosting.

Fig. 3 Sensitivity comparison between TRC and HRC. Sera of immunized mice were tested for HI antibody against H5N1 influenza virus using TRC and HRC separately. Both of the red cells showed HI activity in the sera and HRC provided a higher sensitivity than TRC.
2.5 Anti-H5N1 influenza virus antibody in mouse sera detected by ELISA
ELISA plates were coated with either inactivated H5N1 influenza virus or recombinant M1 (H5N1) protein, and serum samples from immunized mice were added for ELISA experiments. When using inactivated H5N1 influenza virus as the antigen, the OD450value of the M1HA group was significantly greater than the NC group (P<0.05) (Fig. 4), indicating that H5-specific antibodies were present in the sera. There were no significant differences between the M1HA and HA groups (P>0.05). When using M1 protein-coated plates, the OD450value of the M1HA group was significantly greater than the NC group (P<0.05) (Fig. 5), showing the presence of M1 (H5N1)-specific antibodies in mice sera from the M1HA group.
Fig. 4 Detection of anti-influenza virus H5N1 IgG antibody in mouse serum by ELISA. Sera of immunized mice were tested for specific IgG antibody against H5N1 influenza virus by ELISA using inactivated H5N1 influenza virus to coat plates. ELISA results were determined based on the OD450 value.

Fig. 5 Detection of anti-M1 IgG antibody in mouse serum by ELISA. Sera of immunized mice were tested for anti-M1 IgG specific antibody by ELISA using recombinant M1 of H5N1 influenza virus to coat plates. ELISA results were determined based on the OD450 value.
2.6 ELISPOT assay with HA peptide stimulation
In our previous work, synthetic HA peptide fragments, HA-74 (HAaa526-543) and HA-75 (HAaa534-551) have been screened out from HA peptide library used for ELISPOT[10]. Here, HA-74 and HA-75 were used in an ELISPOT assay to detect IFN-γ-secreting cells in the spleens of the immunized mice. The results showed that antigen-specific cellular immune responses were stimulated by both HA-74 and HA-75 peptides and that there were no significant differences between the M1HA and HA groups (P>0.05) (Fig. 6).

Fig. 6 ELISPOT stimulated with peptides of HA. Splenocytes of immunized mice were plated at 2×105 cells per well and HA peptide fragments (HA-74 and HA-75) of H5N1 influenza virus were use to detect IFN-γ-secreting cells.
2.7 ELISPOT assay with M1 peptides stimulation
Four fragments of synthetic M1 peptide, M1-2(M1aa9-26), M1-18(M1aa137-154), M1-19(M1aa145-162) and M1-27(M1aa209-226) were used to stimulate splenocytes in an ELISPOT assay. The results showed that splenocytes from the M1HA group produced more IFN-γ-producing clones than the NC group (Fig. 7), indicating that M1HA immunization successfully stimulated a cellular immune response against the M1 (H5N1) protein.
Fig. 7 ELISPOT stimulated with peptides of M1. Splenocytes of immunized mice were plated at 2×105 cells per well and M1 peptide fragments (M1-2, M1-18, M1-19 and M1-27) of H5N1 influenza virus were use to detect IFN-γ-secreting cells.
3 Discussion
Production of the current inactivated influenza vaccine depends on fertilized chicken eggs. If an influenza pandemic were caused by AIV, the traditional approach of producing influenza vaccines would not be able to meet the global demand. In addition, the HPAIV may cause death of the chicken embryo and not suitable for vaccine production. Therefore, it is widely accepted that novel influenza virus vaccination strategies are urgently needed both to control the spread of HPAIV within species of fowl and to prevent the pandemic spread of HPAIV in humans.
DNA and adenoviral vaccines represent new vaccine strategies that are highly effective in inducing both humoral and cellular immunity and present none of the drawbacks of egg-based vaccines. Furthermore, DNA vaccines and adenoviral vaccines are easy to construct. Antigen elements contained in the vaccines are synthesized in vivo, allowing appropriate posttranslational modifications and presentation to immune system in a natural conformation[11-14]. At present, many studies have shown that immunization with combined DNA and adenoviral vaccines can induce stronger immune response and reduce non-specific reactions caused by viral vectors[15-17]. We therefore used a combined DNA and adenoviral vaccine immunization approach in this study. To enhance the immune response, a combined immunization was used, including the first and third of DNA vaccine immunization, and the second and fourth adenoviral vaccine immunization. HI assay showed a significantly high titer after four immunizations than that after only two immunization. It is suggested that multiple immunization using DNA and adenoviral vaccines may achieve a more desirable immune response.
Gómez-Puertas P, Albo C, et al have demonstrated that co-expression of HA has two effects on the M1 protein: it reduces M1’s tendency to form intracellular tubular structures and it stimulates M1 binding to cell membranes[18]. Therefore, it is helpful to construct a vaccine using a single vector co-expressing the HA and M1 genes. Replication-defective adenovirus has been widely used in vaccine research but the commercial Ad-Easy adenovirus vector expression system provides only one foreign gene insertion site. To co-express two genes using this vector, we constructed a recombinant adenovirus which can co-express M1 and HA genes by IRES insertion into Ad-Easy adenovirus vector. In this study, the recombinant plasmid pStar-M1/HA and recombinant adenovirus Ad-M1/HA containing IRES co-expressing M1 and HA genes were used to immunize mice. In this system, M1 and HA genes can be co-expressed in the same cell, enhancing the synergy between the two proteins. Dual genes co-expression reduces the amount of both the DNA and adenovirus vectors and avoids the competitive inhibition effects when using two separate promoters or transcription factors.
HA is the most important antigenic component of the influenza vaccine but is prone to antigenic shift and antigenic drift, which causes antigenic variation and presents difficulties for influenza prevention and control. In contrast, the M1 protein is well-conserved and can be used as an auxiliary component of an influenza vaccine. Okuda K, et al have demonstrated that immunization with DNA plasmid containing influenza M1 and M2 genes induced protective immunity against influenza virus in mice[19]. Recent research showed that influenza virus matrix protein is the major driving force in virus budding[18,20]and is the only viral component that is essential for VLP formation. VLP vaccines constructed by co-expression of M1 and HA proteins have shown a promising immunization effects in animals[21-22]. Therefore, immunization by co-expressing M1 and HA genes is a relevant approach. The results in this study showed that humoral and cellular immune response against M1 and HA proteins were stimulated by vectors co-expressing the M1 and HA genes. Furthermore, when compared with a group immunized with the HA gene alone, humoral and cellular immune response to HA protein in the M1HA group were not diminished. This finding suggests that co-immunization with M1 and HA genes may provide better immune protection than single HA gene immunization. Studying the immune response to recombinant DNA and adenoviral vaccines co-expressing M1 and HA genes of H5N1 influenza virus type A provides a new way to research and develop novel influenza vaccines.
REFERENCES
[1] Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet, 1998, 351(9101): 472−477.
[2] Subbarao K, Klimov A, Katz J, et al. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science, 1998, 279(5349): 393−396.
[3] Yu H, Shu Y, Hu S, et al. The first confirmed human case of avian influenza A (H5N1) in Mainland China. Lancet, 2006, 367(9504): 84.
[4] Helenius A. Unpacking the incoming influenza virus. Cell, 1992, 69(4): 577−578.
[5] Watanabe K, Handa H, Mizumoto K, et al . Mechanism for inhibition of influenza virus RNA polymerase activity by matrix protein. J Virol, 1996, 70(1): 241−247.
[6] Nayak DP, Hui EKW, Barman S. Assembly and budding of influenza virus. Virus Res, 2004, 106(2): 147−165.
[7] Holterman L, Vogels R, van der Vlugt R, et al. Novel replicationincompetent vector derived from adenovirus type 11 (Ad11) for vaccination and non-cross-reactivity with Ad5. J Virol, 2004, 78(23): 13207−13215.
[8] Gao WT, Soloff AC, Lu XH, et al. Protection of mice and poultry from lethal H5N1 avian influenza virus through adenovirus-based immunization. J Virol, 2006, 80(4): 1959−1964.
[9] Hoeslscher MA, Garg S, Bangari DS, et al. Development of adenoviral-vector-based pandemic influenza vaccine against antigenically distinct human H5N1 strains in mice. Lancet, 2006, 367(9509): 475−481.
[10] Guo JQ, Yao LH, Chen AJ, et al. Immunological evaluation of vector-expressed M2 and HA genes of H5N1 influenza virus in mice. Chin J Biotech, 2010, 26(5): 649−656.郭建强, 姚立红, 陈爱珺, 等. 甲型H5N1流感病毒M2与HA双基因载体疫苗在小鼠体内的免疫学评价. 生物工程学报, 2010, 26(5): 649−656.
[11] Ulmer JB, Donnelly JJ, Parker SE, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science, 1993, 259(5102): 1745−1749.
[12] Chen MW, Cheng TJ, Huang YX, et al. A consensushemagglutinin-based DNA vaccine that protects mice against divergent H5N1 influenza viruses. Proc Natl Acad Sci USA, 2008, 105(36): 13538−13543.
[13] Van Kampen KR, Shi Z, Gao P, et al. Safety and immunogenicity of adenovirus-vectored nasal and epicutaneous influenza vaccines in humans. Vaccine, 2005, 23(8): 1029−1036.
[14] Toro H, Tang DCC, Suarez DL, et al. Protection of chickens against avian influenza with non-replicating adenovirus-vectored vaccine. Vaccine, 2008, 26(21): 2640−2646.
[15] Xiang ZQ, Pasquini S, Ertl HC. Induction of genital immunity by DNA priming and intranasal booster immunization with a replication-defective adenoviral recombinant. J Immunol, 1999, 162(11): 6716−6723.
[16] Koup RA, Roederer M, Lamoreaux L, et al. Priming immunization with DNA augments immunogenicity of recombinant adenoviral vectors for both HIV-1 specific antibody and T-cell responses. PLoS ONE, 2010, 5(2): e9015.
[17] Cox KS, Clair JH, Prokop MT, et al. DNA gag/adenovirus type 5 (Ad5) gag and Ad5 gag/Ad5 gag vaccines induce distinct T-cell response profiles. J Virol, 2008, 82(16): 8161−8171.
[18] Gómez-Puertas P, Albo C, Pérez-Pastrana E. Influenza virus matrix protein is the major driving force in virus budding. J Virol, 2000, 74(24): 11538−11547.
[19] Okuda K, Ihata A, Watabe S, et al. Protective immunity against influenza A virus induced by immunization with DNA plasmid containing influenza M gene. Vaccine, 2001, 19(27): 3681−3691.
[20] Ali A, Avalos RT, Ponimaskin E, et al. Influenza virus assembly: effect of influenza virus glycoproteins on the membrane association of M1 protein. J Virol, 2000, 74(18): 8709−8719.
[21] Quan FS, Huang CZ, Compans RW, et al. Virus-like particle vaccine induces protective immunity against homologous and heterologous strains of influenza virus. J Virol, 2007, 81(7): 3514−3524.
[22] Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a Lethal Influenza virus challenge. Viral Immunol, 2005, 18(1): 244−251.
