三维超分子化合物[Cu2(phen)2(H2O)4]·(SO4)2的水热合成、晶体结构及荧光性质研究
,,,奎蓉,
(1.延边大学 理学院 化学系,吉林 延吉 133002;2.淮阴师范学院 化学化工学院,江苏 淮安 223300)
0 引言
近年来,配位聚合物由于其迷人的结构及在催化、吸附、电子传输、磁性、光学性等领域的潜在应用,引起国内外研究人员的广泛关注[1-6].水热合成对于配位聚合物的制备是一种有效且简单的合成方法,其起始反应物通常为简单的无机物和有机物,一些具有新奇结构的配位聚合物大多数是由水热法合成得到[7-11].邻菲罗啉具有刚性、平面性和芳香性,在配位化学领域是一个很好的结构构筑模块,其作为一种配位能力很强的二齿配体已被广泛应用于金属-有机配位聚合物的合成,如Cu(II)离子与有机配体phen合成的配位聚合物[12-16],但在已报道的Cu(II)-phen化合物中具有一维交叉结构的相对较少.本文中,我们报道了水热条件下基于静电引力、氢键和π—π堆积等弱的分子间作用力由一维交叉结构构筑而成的三维超分子[Cu2(phen)2(H2O)4]·(SO4)2(化合物1),同时研究了室温下化合物1的固体荧光性质.
1 实验部分
1.1 试剂与仪器
2400II型元素分析仪(美国PE公司);SMART APEXII CCD单晶X-射线衍射仪(德国Bruker公司),钼靶,λ=0.071073nm;ARL X`TRA型粉末X-射线衍射仪(瑞士Thermo公司),铜靶,λ=0.15418nm;AVATAR360型红外光谱仪(美国Nicolet公司),KBr压片,波数范围4000~400cm-1;固态样品荧光光谱是在Perkin-Elmer LS55型荧光光谱仪(美国PE公司)上于室温下测定.所用试剂中哌嗪膦酸根据文献合成[17].其他均为市售分析纯,水为蒸馏水.
1.2 化合物1的合成
称取0.6mmol (0.150g)CuSO4·5H2O、0.8mmol (0.160g)邻菲罗啉和0.6mmol (0.165g)哌嗪膦酸于25mL烧杯中,加入5mL去离子水,在室温下搅拌0.5h.把混合物移到23mL内衬为聚四氟乙烯的不锈钢水热反应釜中,放入140℃烘箱内,在自生压力条件下反应4天后取出.冷却至室温,经过滤、去离子水洗涤于室温下晾干,得黄绿色晶体,产率30%(基于Cu计算).元素分析实验值(%): C 38.19,H 3.091,N 7.342;理论值(%): C 38.31,H: 3.193,N 7.450.IR分析(KBr压片,cm-1): 3129 (m),1620 (w),1400 (m),1088 (m),614 (w),474 (m).
1.3 晶体结构的测定
选取0.030mm×0.020mm×0.015mm的单晶,利用SMART PEX II CCD单晶衍射仪进行衍射数据收集.在296(2)K下用Mo-Kα射线(λ=0.071073nm),以multi-scan扫描方式在1.69°≤θ≤25.00°范围内共收集13946个衍射点,其中3614个独立衍射点和3594个可观测衍射点[I>2σ(I)]用于结构分析和结构修正.晶体结构采用SHELXS-97程序由直接法解出,结构精修采用SHELXS-97程序,对氢原子和非氢原子分别采用各向同性和各向异性温度因子进行全矩阵最小二乘法修正.相应的晶体学数据及结构修正数据见表1.

表1 化合物1的晶体学数据
2 结果与讨论
2.1 晶体结构描述
如图1所示,化合物1的不对称结构单元中包含2个晶体学独立的二价铜离子、2个phen有机

图1 化合物1的分子结构

表2 化合物1的部分键长(nm)和键角

表3 化合物1的氢键键长(nm)和键角
对称操作: #1:-x,y,-z+1/2;#2:-x,y,-z-1/2;#3:-x,-y+1,-z;#4: x,-y,z+1/2;#5:-x,y,1/2-z;#6:x,1-y,1/2+z;#7: 1/2-x,1/2-y,-z
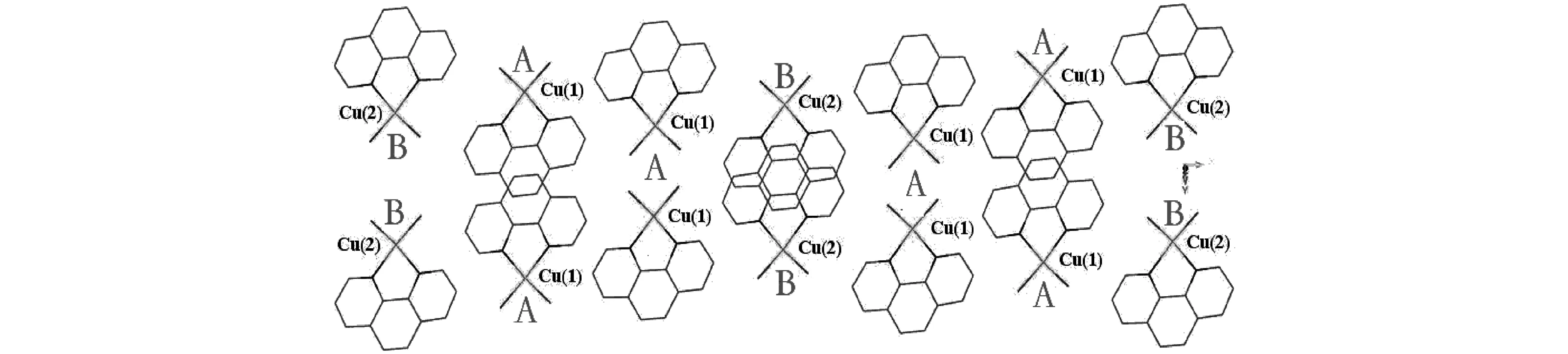
如图2所示,以Cu(1)为中心的配位单元[Cu(1)N2O2]2+为A,以Cu(2)为中心的配位单元[Cu(2)N2O2]2+为B.各配位单元在众多氢键的连接作用下,O(7)—H(72)…O(9)(0.264(2)nm,147.8°),O(7)—H(71)…O(8)(0.319(2)nm,120.3°),O(2)—H(21)…O(5)(0.267(2)nm,133.1°),O(1)—H(11)…O(4)(0.263(2)nm,115.3°),C(14)—H(14)…O(5)(0.334nm,152.0),C(9)—H(9)…O(9)(0.333nm,146.0°),C(5)—H(5)…O(6)(0.324nm,136.0°),C(2)—H(2)…O(4)(0.338nm,144.0°),以BAA的模式进行交替排列,沿[100]面扩展形成一维梯层状链,如图3.该一维链继续在氢键O(7)—H(72)…O(8)(0.275(3)nm,124.9°),O(7)—H(71)…O(9)(0.269(2)nm,111.9°),O(2)—H(21)…O(1)(0.2.89(2)nm,122.9°),O(1)—H(11)…O(5)(0.347(2)nm,173.9°),O(1)—H(11)…O(2)(0.289(2)nm,113.0°)的连接作用下,以及芳环间的错位π—π堆积和面对面π—π堆积的作用下被构筑成了三维超分子结构,如图4所示.相应的π—π堆积作用分别发生在六元环(N(1)—C(1)—C(2)—C(3)—C(4)—C(12))与(C(4)—C(5)—C(6)—C(7)—C(11)—C(12))之间(面心距为0.3941nm,二面角为1.08°)、六元环(N(2)—C(10)—C(9)—C(8)—C(7)—C(11))与(C(4)—C(5)—C(6)—C(7)—C(11)—C(12))之间(面心距为0.3935nm,二面角为0.59°)、六元环(C(4)—C(5)—C(6)—C(7)—C(11)—C(12))与(C(4)—C(5)—C(6)—C(7)—C(11)—C(12))之间(面心距为0.3534nm,二面角为0.33°)、六元环(N(3)—C(13)—C(14)—C(15)—C(16)—C(17))与(C(16)—C(17)—C(17A)—C(16A)—C(18A)—C(18))之间(面心距为0.3946nm,二面角为2.00°)以及六元环(C(16)—C(17)—C(17A)—C(16A)—C(18A)—C(18))与(C(16)—C(17)—C(17A)—C(16A)—C(18A)—C(18))之间(面心距为0.3531nm,二面角为0.00°).化合物1的氢键键长和键角见表3.
(图中为清晰起见删除了游离的离子)

图3 沿[100]面配位单元[CuN2O2]2+以BAA方式排列与离子通过氢键作用形成的一维梯层状链

图4 化合物1的三维超分子结构图
2.2 粉末XRD表征
对化合物1进行了粉末X射线衍射分析(Cu Kα辐射,λ=0.15418nm),并根据单晶X射线衍射所得数据,利用Powdercell For Windows v2.4(简称PCW)软件模拟了该化合物的粉末X射线谱图.实验所得谱图中的衍射峰与模拟谱图中的衍射峰的位置基本吻合(图 5),表明化合物是纯相.而衍射强度的不同主要是由粉末样品在收集衍射数据时首选的取向不同所造成.
2.3 荧光性质研究
室温下,对化合物1的固态荧光性质进行了探讨.固体荧光测试结果表明化合物1在270nm的激发波长下于450nm波长处有一个荧光发射峰,如图 6 所示.据文献报道[21],自由配体phen在310nm激发波长下,在365nm和388nm两个波长处出现可归为分子内π*—π电荷转移的荧光发射峰.所以化合物1在450nm处的荧光发射峰可能是由配体到金属间的电荷转移(LMCT)或者金属到配体间的电荷转移(MLCT)所致.

图5 化合物1实验(a)与模拟(b)粉末XRD图

图6 化合物1的室温固体荧光谱图
3 结论

[1]Reineke T M,Eddaoudi M,Fehr M,et al.From Condensed Lanthanide Coordination Solids to Microporous Frameworks Having Accessible Metal Sites[J].J Am Chem Soc,1999,121(8): 1651-1657.
[2]Biradha K,Seward C,Zaworotko M J.Helical coordination polymers with large chiral cavities[J].Angew Chem Int Ed,1999,38(4): 492-495.
[3]Ma K R,Xu J N,Zhang P,et al.Self-assembly,crystal structure and photoluminescent properties of a novelorganic-inorganic hybrid coordination polymer: [CdCl3(CH3)3NH][J].Solid State Sciences,2006,8(12): 1473-1476.
[4]Ma K R,Zhu Y L,Yin Q F.Solvothermal synthesis and characterization of a new ZnII-PMIDA phosphonate[J].J Coord Chem,2009,62(20): 3243-3249.
[5]Gamez P,Hoog P,Roubeau O,et al.An unprecedented 1D ladder coordination polymer based on a pentanuclear copper(II)2,4,6-tris(dipyridin-2-ylamino)-1,3,5-triazine building block[J].Chem Commun,2002,14: 1488-1489.
[6]Shi Z,Feng S,Sun Y,et al.Novel coordination polymers with mixed ligands and orientated enantiomers[J].Inorg Chem,2001,40(21): 5312-5313.
[7]Hagrman P J,Hagrman D,Zubieta J,et al.Organic-inorganic hybrid materials: From “simple” coordination polymers to organodiamine-templated molybdenum oxides[J].Angew Chem Int Ed,1999,38(18): 2639-2684.
[8]Hagrman D,Hausualter R C,Zubieta J.Three-dimensional organic/inorganic hybrid materials constructed from one-dimensional copper diamine coordination polymers linked by bridging oxoanion tetrahedra: [Cu(dpe)(MoO4)]and [Cu(dpe)(SO4)(H2O)](dpe=1,2-trans-(4-pyridyl)ethene)[J].Chem Mater,1998,10(1): 361-365.
[9]Tao J,Zhang Y,Tong M,et al.A mixed-valence copper coordination polymer generated by hydrothermal metal/ligand redox reactions[J].Chem Commun,2002,13: 1342-1343.
[10]Lu J Y,Babb A M.The first triple-layer 2-D coordination polymer:[Cu3(bpen)(IN)6(H2O)2][J].Inorg Chem,2001,40(14): 3261-3262.
[11]Zhu Y L,Tang X L,Ma K R,et al.Synthesis,Structures and Photoluminescent Properties of Two Novel Zinc(II)Compounds Constructed from 5-Sulfoisophthalic Acid[J].Bull Korean Chem Soc,2010,31(7): 1881-1886.
[12]Ross S A,Pitie M,Meunier B.Synthesis of two acridine conjugates of the bis(phenanthroline)ligand “Clip-Phen” and evaluation of the nuclease activity of the corresponding copper complexes[J].Eur J Inorg.Chem,1999,1999(3): 557-563.
[13]Korpi H,Figiel P J,Lankinen E,et al.On in situ prepared Cu-phenanthroline complexes in aqueous alkaline solutions and their use in the catalytic oxidation of veratryl alcohol[J].Eur J Inorg Chem,2007,2007(17): 2465-2471.
[14]Zaworotko M J.Superstructural diversity in two dimensions: crystal engineering of laminated solids[J].Chem Commun,2001,01: 1-9.
[15]Clifford F,Counihan E,Fitzgerald W,et al.The Crystal Structures of[Cu(phen)2(O2CMe)]X (phen=1,10-phenanthroline)Complexes: Pseudo cis-Distorted Octahedral Structures and Fluxional Copper(II)Stereochemistries[J].Chem Commun,1982,1982(3): 196-198.
[16]Simmons C J,Alcock M W,Seff K,et al.A Fluxional Pseudo-Jahn-Teller Complex: The Structure of (Acetato)bis(1,10-phenanthroline)copper(lI)Perchlorate,[Cu(C12H8N2)2(C2H302)]ClO4,at 298 and 173 K[J].Acta Cryst, 1985,B(41): 42-46.
[17]Groves J A,Wright P A,Lightfoot P.Two Closely Related Lanthanum Phosphonate Frameworks Formed by Anion-Directed Linking of Inorganic Chains[J].Inorg Chem,2005,44(6): 1736-1739.
[18]Server-Carrio J,Escriva E,Folgado J.Crystal and molecular structure and electronic properties of [Cu(phen)2(HL)]·(phen)0.5·7H2O (H3L=1,3,5-triazine-2,4,6(1H,3H,5H)-trione),a novel N-cyanurate(2-)derivative[J].Polyhedron,1998,17(9): 1495-1501.
[19]McCann M,Humphreys F,McKee V.Transition metal complexes of dibenzoyl-L-tartaric acid (db-L-tarH2)and L-tartaric acid (L-tarH2);X-ray crystal structure of {[Cu(L-tar)(phen)]·6H2O}n(phen=1,10-phenanthroline)[J].Polyhedron,1997,16(20): 3655-3661.
[20]Youngme S,Wannarit N,Pakawatchai C,et al.Structural diversities and spectroscopic properties of bis and tris(1,10-phenanthroline)copper(II)complexes[J].Polyhedron,2007,26(7): 1459-1468.
[21]Shi X,Zhu G S,Fang Q R,et al.Novel Supramolecular Frameworks Self-Assembled from One-Dimensional Polymeric Coordination Chains[J].Eur J Inorg Chem,2004,5(1): 185-191.

