四个桂花品种挥发油成分研究
康文艺,王金梅
河南大学中药研究所,开封 475004
Introduction
The genusOsm anthusLour.belongs to the family Oleaceae and was established in 1970[1].There are about 30 species in the genusOsm anthusin Northern A-merica,Eastern Europe,South-eastAsia and the Pacific Islands[2],but only one species,i.e.O.fragrans as a flower native to China,is cultivated extensively and has long been prized for its abundant s weet-scented flowers[3].As one of ten top traditional flowers in China,O. fragransis especially valued as an additive for tea,other beverages and also as an economic plant in the east. O.fragrans’extracts are of high value and accordingly are used in only the most expensive perfumes and flavours,but is mostly used for aromatic therapy[4].In addition,O.fragransinmedicinal useswith an anti-tussive of its flowers and its essential oil used as a flavouring[5].During the long time of evolution,O.fragrans varies abundant variants under influences of natural and artifical selects,which are classified into four groups(Fragrans Group,Latifolius Group,Thunbergii Group,and Aurantiacus Group)according to morphological characteristics[6].Because of the insufficiency in phylogenetic study,the work of cultivar classification mainly accordsto morphological and physiological characters,and it can’t produce subject conclusion.In order to ensure smooth running of International Registration Authority ofO.fragransCultivars and provide evidence for taxonomy ofO.fragrands,we studied four O.fragranscultivars,belong to four groups,by HSSPME-GC-MC combined with Kovats index.
Materials andM ethods
PlantM aterials
The flowers ofO.fragrans“Baijie” (Latifolius Group),O.fragrans“Baiyudangui” (Aurantiacus Group) andO.fragrans“Fodingzhu” (Fragrans Group)were collected from Shanghai,China,andO. fragrans“Jingui”(Thunbergii Group)were collected from Guilin,Guangxi province,China,in October 2006.All samples were dried in the shade(at room temperature).They were identified by Pro.Fude Sang (Institute of Natural Products,Henan University),and a voucher specimen was deposited in the Institute of Natural Products,Henan University,Kaifeng,China.
Solid-phaseM icroextraction(SPM E)
Volatiles were extracted by the manual SPME holder together with 5 mL vials and PDMS-DVB fibers purchased from Supelco Inc.(Bellefonte,USA).Prior to extraction,the fiberwas activated for 10 min in the GC 6875(Agilent USA)with an injector temperature of 250℃.The powder of flowers and buds about 0.7 g was placed in vials(5 mL),then the SPME fiber was exposed in the upper space of the sealed vial for 30 min at 80℃to adsorb the analytes.After that,the fiber waswithdrawn and directly inserted into the GC-MS inlet for desorption of the volatiles for 1 min.
GC-MS Analysis Conditions
The volatile constituentswere analyzed by GC-MS.The GC-MS analyses were performed in a GC-MS(gas chromatograph 6890 N coupled to a selective detector mass spectrometer 5975 inert,Agilent Technologies). Compound separation was carried out using a capillary column HP-5 MS(30 m×0.25 mm,0.25μm fi lm thickness).Helium was used as carrier gas(1 mL/ min).The front inlet was kept at 250℃ in split-less mode.The temperature program as below:the initial column temperature was 50℃,held for 1 min;and then programmed to 120℃ at a rate of 3℃/min and held for 2 min;finally programmed to 210℃at a rate of 4℃/min,held at 210℃for 10 min.TheMS detectorwas used in the EImode with an ionization voltage of 70 eV.The ion source temperature was at 230℃. The transfer line was at 280℃.The spectra were collected at 3 scans/s over the mass rang(m/z)30-440. Identification of com pounds
The compoundswere identified by comparison with the Rtlpest3.L and Nist05.L mass spectral database.Kovats index(KI)of the sample componentswere determined on the basis of C6-C26n-alkanes(Alfa Aesar) detected under the same conditions.The quantitative composition was obtained by peak area normalization, and the response factor for each componentwas considered to equal to 1.Some compoundswere identified by comparing their retention indices and mass spectra with published in literatures.
Results and discussion
72 componentswere identified from“Baijie”,“Baiyudangui”,“Fodingzhu”and“Jingui”and listed in Table 1 with their percentage compositions.The constituents are listed in order of their elution from HP-5MS column.45 compounds were identified from“Baijie”, representing about 67.30%of the total composition, with a high content ofα-methyl-α-[4-methyl-3-pentenyl]oxiranemethanol(14.63%),followed by 2,6-d imethyl-2,7-octadiene-1,6-diol(3.96%)and hexadecane(3.82%).45 compoundswere identified from Baiyudangui,representing about 66.18%of the total composition,with a high content ofα-methyl-α-[4-methyl-3-pentenyl]oxiranemethanol(15.24%),followed by 2,6,10-trimethyl-hexadecane(3.64%)and 3,7-dimethyl-1,6-octadien-3-ol(3.57%).40 compoundswere identified from“Fodingzhu”,representing about 69.54%of the total composition,with a high content ofα-methyl-α-[4-methyl-3-pentenyl] oxiranemethanol(14.68%),followed by 3,7-dimethyl-1,6-octadien-3-ol(9.83%)and 3-vinyl-3-methyl-cyclohexanone(5.17%).46 compounds were identified from“Jingui”,representing about 81.01%of the total composition,with a high content of diisobutyl phthalate (23.99%),followed by di-n-butylphthalate(4.30%) and 5-hexyldihydro-2(3H)-furanone(3.96%).
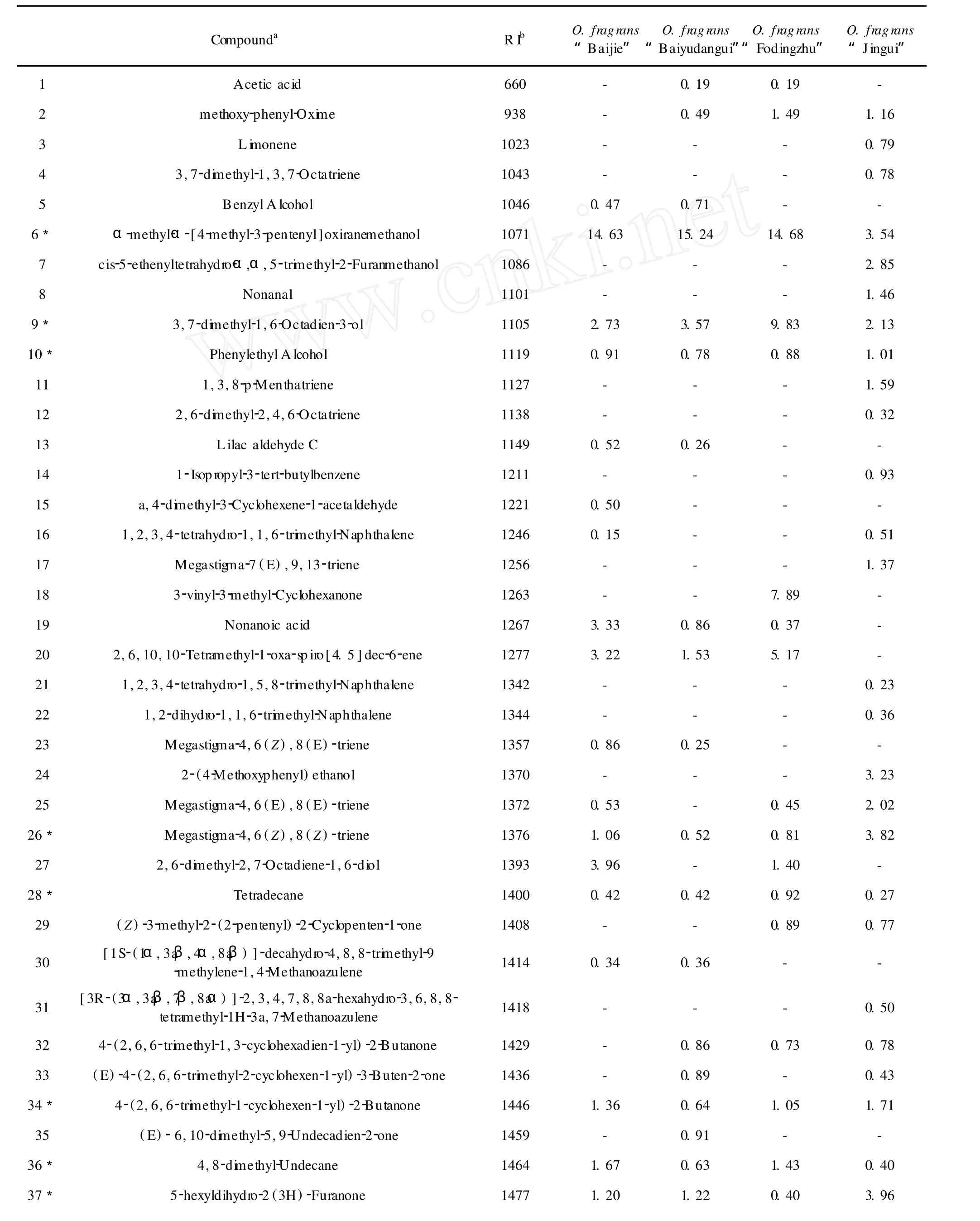
Table 1 The volatile constituents from four genus ofO.fragranscultivars
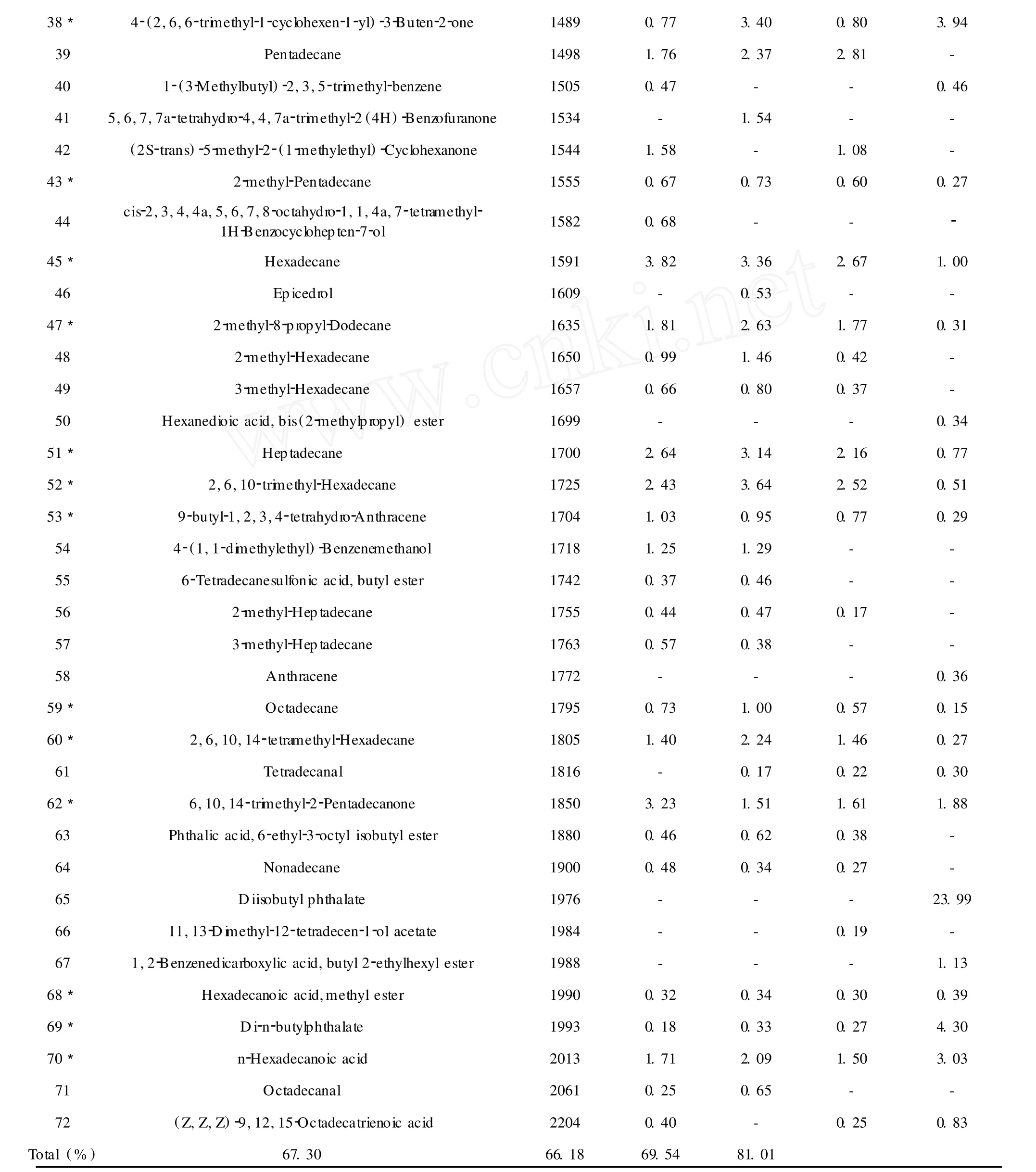
aCompounds listed in orderof their KI.bKI(Kovats index)measured relative to n-alkanes(C6-C26)on a nonpolarHP-5MS column.%,Relative percentage obtained from peak area.*,Compound in all samples.
In Table 1,21 compounds were mutual in fourO.fragranscultivars,such asα-methyl-α-[4-methyl-3-pentenyl]oxiranemethanol,3,7-d imethyl-1,6-octadien-3-ol,phenylethyl alcohol,megastigma-4,6(Z),8(Z)-triene and so on.However,there are some differences among these four samples.For example,α-methyl-α-[4-methyl-3-pentenyl]oxiranemethanol,the main compound in“Baijie”,“Baiyudangui”and“Fodingzhu”, was decreased to 3.94%in“Jingui”,and 3,7-d imethyl-1,6-Octadien-3-ol(9.83%)was the highest in“Fodingzhu”among these four samples.3-vinyl-3-methyl-Cyclohexanone(7.89%)was only detected in“Fodingzhu”,while diisobutyl phthalate(23.99%) was only detected in“Jingui”and so on.
The results showed that there was a significant correlation between the chemical constituents inO.fragrans, and chemical constituentsmight be anxiliary identification of variants ofO.fragrans.
Acknwledgment Thiswork was supported by the foundation of basic project of Henan Provincial Office of Education(2008A360002)and Henan Province Office of Education teachers,the backbone of Youth Fund (2008).
1 Liu LC,XiangQB.Research progress onOsm anthus genus.J Nanjing Forestry Univ,Nat Sci,2003,27:84-88.
2 Kubba A,Tillequin F,Koch M,et al.Iridoids,lignan,and triterpenes fromOsmanthus cymosus.B iochem Syst Ecol. 2005,33:305-307.
3 Zang DK,Xiang QB.Studies onOsm anthus fragranscultivars.J Nanjing Forestry Univ,Nat Sci,2004,28(S1):7-13.
4 Pan Y M,Zhu ZR,Huang ZL,et al.Characterisation and free radical scavenging activities of novel red pigment from Osmanthus fragrans’seeds.Food Chem istry,2009,112:909-913.
5 Lee HH,Lin CT,Yang LL.Neuroprotection and free radical scavenging effects of Osmanthus fragrans.J B iom ed Sci, 2007,14:819-827.
6 ZangDK,Xiang QB,Liu YL.Notes on cultivar classification inOsm anthus.Scientia Silvae Sinicae,2006,42,17-21.